Difference between revisions of "PFAS Treatment by Electrical Discharge Plasma"
(Tag: Visual edit) |
|||
| (7 intermediate revisions by the same user not shown) | |||
| Line 6: | Line 6: | ||
*[[Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS)]] | *[[Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS)]] | ||
*[[PFAS Ex Situ Water Treatment]] | *[[PFAS Ex Situ Water Treatment]] | ||
| + | *[[PFAS Soil Remediation Technologies]] | ||
| + | *[[PFAS Sources]] | ||
| + | *[[PFAS Transport and Fate]] | ||
| + | *[[Photoactivated Reductive Defluorination - PFAS Destruction]] | ||
| + | *[[Supercritical Water Oxidation (SCWO)]] | ||
| − | |||
| − | + | '''Contributor(s):''' [[Dr. Selma Mededovic Thagard]], [[Dr. Thomas Holsen]], [[Dr. Stephen Richardson |Dr. Stephen Richardson, P.E]], [[Poonam Kulkarni |Poonam Kulkarni, P.E.]] and Dr. Blossom Nzeribe | |
| − | + | ||
| − | |||
| − | |||
| − | |||
'''Key Resource(s):''' | '''Key Resource(s):''' | ||
| − | *[https://pfas-1.itrcweb.org/12-treatment-technologies/#12_2 | + | *Interstate Technology Regulatory Council (ITRC), PFAS – Per- and Polyfluoroalkyl Substances: [https://pfas-1.itrcweb.org/12-treatment-technologies/#12_2 12.2 Field-Implemented Liquids Treatment Technologies] and [https://pfas-1.itrcweb.org/12-treatment-technologies/#12_5 12.5 Limited Application and Developing Liquids Treatment Technologies]. |
| − | *Physico-Chemical Processes for the Treatment of Per- And Polyfluoroalkyl Substances (PFAS): A | + | *Physico-Chemical Processes for the Treatment of Per- And Polyfluoroalkyl Substances (PFAS): A review<ref name="Nzeribe2019">Nzeribe, B.N., Crimi, M., Mededovic Thagard, S. and Holsen, T.M., 2019. Physico-Chemical Processes for the Treatment of Per- And Polyfluoroalkyl Substances (PFAS): A review. Critical Reviews in Environmental Science and Technology, 49(10), pp.866-915. [https://doi.org/10.1080/10643389.2018.1542916 DOI: 10.1080/10643389.2018.1542916]</ref> |
*Low Temperature Plasma for Biology, Hygiene, and Medicine: Perspective and Roadmap<ref name="Laroussi2021">Laroussi, M., Bekeschus, S., Keidar, M., Bogaerts, A., Fridman, A., Lu, X.P., Ostrikov, K.K., Hori, M., Stapelmann, K., Miller, V., Reuter, S., Laux, C., Mesbah, A., Walsh, J., Jiang, C., Mededovic Thagard, S., Tanaka, H., Liu, D.W., Yan, D., and Yusupov, M., 2021. Low Temperature Plasma for Biology, Hygiene, and Medicine: Perspective and Roadmap. IEEE Transactions on Radiation and Plasma Medical Sciences. [https://doi.org/10.1109/TRPMS.2021.3135118 DOI: 10.1109/TRPMS.2021.3135118] [https://ieeexplore.ieee.org/stamp/stamp.jsp?tp=&arnumber=9650590 Article pdf]</ref> | *Low Temperature Plasma for Biology, Hygiene, and Medicine: Perspective and Roadmap<ref name="Laroussi2021">Laroussi, M., Bekeschus, S., Keidar, M., Bogaerts, A., Fridman, A., Lu, X.P., Ostrikov, K.K., Hori, M., Stapelmann, K., Miller, V., Reuter, S., Laux, C., Mesbah, A., Walsh, J., Jiang, C., Mededovic Thagard, S., Tanaka, H., Liu, D.W., Yan, D., and Yusupov, M., 2021. Low Temperature Plasma for Biology, Hygiene, and Medicine: Perspective and Roadmap. IEEE Transactions on Radiation and Plasma Medical Sciences. [https://doi.org/10.1109/TRPMS.2021.3135118 DOI: 10.1109/TRPMS.2021.3135118] [https://ieeexplore.ieee.org/stamp/stamp.jsp?tp=&arnumber=9650590 Article pdf]</ref> | ||
| Line 66: | Line 67: | ||
==See Also== | ==See Also== | ||
| + | [https://soundcloud.com/arcadis-north-america/plasma-destruction-of-pfas-in-groundwater?utm_source=clipboard&utm_campaign=wtshare&utm_medium=widget&utm_content=https%253A%252F%252Fsoundcloud.com%252Farcadis-north-america%252Fplasma-destruction-of-pfas-in-groundwater SERDP and ESTCP PFAS Reserach and Remediation Podcast : Plasma Destruction of PFAS in Groundwater] | ||
Latest revision as of 19:09, 31 October 2024
Plasma-based water treatment is a technology that, using only electricity, converts water into a mixture of highly reactive species including OH•, O, H•, HO2•, O2•‒, H2, O2, H2O2 and aqueous electrons (e‒aq), called a plasma[1][2]. These highly reactive species rapidly and non-selectively degrade volatile organic compounds (VOCs)[3], 1,4-Dioxane[4][5], and a broad spectrum of per- and polyfluoroalkyl substances (PFAS) including perfluorooctanoic acid (PFOA), perfluorooctane sulfonic acid (PFOS), and short-chain PFAS[6][7][8]. A plasma reactor can simultaneously oxidize and reduce organics by producing a mixture of hydroxyl radicals and aqueous electrons, the latter of which act as strong reducing agents and could be the key species in removing PFAS and other non-oxidizable compounds. Additionally, the plasma process produces no residual waste and requires no chemical additions, although adding surfactants or injecting inert gas into the liquid phase can increase interfacial PFAS concentrations, exposing more of the PFAS to the plasma and therefore increasing removal efficiency.
Related Article(s):
- Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS)
- PFAS Ex Situ Water Treatment
- PFAS Soil Remediation Technologies
- PFAS Sources
- PFAS Transport and Fate
- Photoactivated Reductive Defluorination - PFAS Destruction
- Supercritical Water Oxidation (SCWO)
Contributor(s): Dr. Selma Mededovic Thagard, Dr. Thomas Holsen, Dr. Stephen Richardson, P.E, Poonam Kulkarni, P.E. and Dr. Blossom Nzeribe
Key Resource(s):
- Interstate Technology Regulatory Council (ITRC), PFAS – Per- and Polyfluoroalkyl Substances: 12.2 Field-Implemented Liquids Treatment Technologies and 12.5 Limited Application and Developing Liquids Treatment Technologies.
- Physico-Chemical Processes for the Treatment of Per- And Polyfluoroalkyl Substances (PFAS): A review[9]
- Low Temperature Plasma for Biology, Hygiene, and Medicine: Perspective and Roadmap[10]
Introduction
Plasma processing plays an essential role in various industrial applications such as semiconductor fabrication, polymer functionalization, chemical synthesis, agriculture and food safety, health industry, and hazardous waste management[11][12][13][14]. Plasma is a gaseous state of matter consisting of charged particles, metastable-state molecules or atoms, and free radicals. Depending on the energy or temperature of the electrons, compared with the temperature of the background gas, plasmas can be classified as thermal or non-thermal. In thermal plasma, an example of which is an electrical arc, individual species’ temperatures typically exceed several thousand Kelvins (K). Non-thermal plasmas are formed using less power with temperatures ranging from ambient to approximately 1000 K[15]. An example of a non-thermal plasma is a dielectric barrier discharge used for commercial ozone generation.
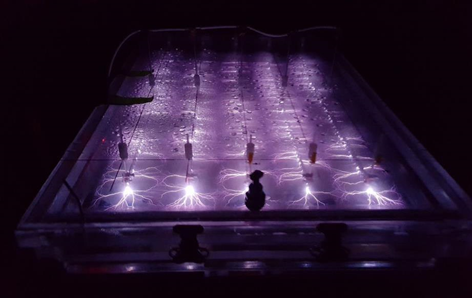
Plasma that is applied in water treatment (Figure 1) is typically non-thermal, which offers high-energy process efficiency and selectivity[15][16]. Since the 1980s when the first plasma reactor was utilized to oxidize a dye[17], over a hundred different plasma reactors have been developed to treat a range of contaminants of environmental importance including biological species. Examples include treatment of pharmaceuticals, volatile organic compounds (VOCs), 1,4-dioxane, herbicides, pesticides, warfare agents, bacteria, yeasts and viruses using direct-in-liquid discharges with and without bubbles and discharges in a gas over and contacting the surface of a liquid. Different excitation sources including AC, nanosecond pulsed and DC voltages have been utilized to produce pulsed corona, corona-like, spark, arc, and glow discharges, among other discharge types. Many reviews of plasma processing for water treatment applications have recently been published[18][19].
Plasma-based water treatment (PWT) owes its strong oxidation and disinfection capabilities to the production of reactive oxidative species (ROS), primarily OH radicals, atomic oxygen, singlet oxygen and hydrogen peroxide. The process also produces reductive species such as solvated electrons and reactive nitrogen species (RNS) when nitrogen and oxygen are present in the discharge. This process has the advantage of synergistic effects of high electric fields, UV/VUV light emissions and in some cases shockwave formation in a liquid. It requires no chemical additions, and can be optimized for batch or continuous processing.
Application of Plasma for the Treatment of PFAS-Contaminated Water
Several research groups have investigated the use of plasma to treat and remove PFAS from contaminated water[20][21][22][23][24][25][26][27][28]. Of those studies, the Enhanced Contact (EC) plasma reactor developed by researchers at Clarkson University is one of the most promising in terms of treatment time, cost, the range of PFAS treated and scale up/throughput. Their process has been shown to degrade PFOA, PFOS, and other PFAS in a variety of PFAS-impacted water sources.
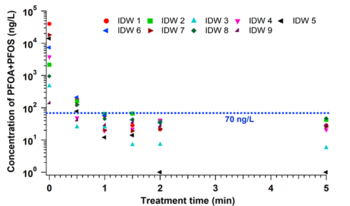
In the EC plasma reactor (Figure 2), argon gas is continuously pumped through the solution to form a layer of foam and thus concentrate PFAS at the gas-liquid interface where plasma is formed. The process is able to lower the concentrations of PFOA and PFOS in groundwater obtained from multiple DoD sites to below Environmental Protection Agency’s (EPA’s) lifetime health advisory level (HAL) of 70 parts per trillion (70 nanogram per liter, ng/L)[29] within 1 minute of treatment (Figure 3) with energy requirements much lower than those of alternative technologies (~2-6 kWh/m3 for plasma vs. 5000 kWh/m3 for persulfate, photochemical oxidation and sonolytic processes and 132 kWh/m3 for electrochemical oxidation)[7][9]. The EC plasma reactor owes its high efficacy to the plasma reactor design, in particular to the gas bubbling through submerged diffusers to transport PFAS to the plasma-liquid interface and thus minimize bulk liquid limitations.
In 2019, a mobile plasma treatment system (Figure 4) was successfully demonstrated for the treatment of PFAS-contaminated groundwater at the fire-training area at Wright-Patterson Air Force Base[30].
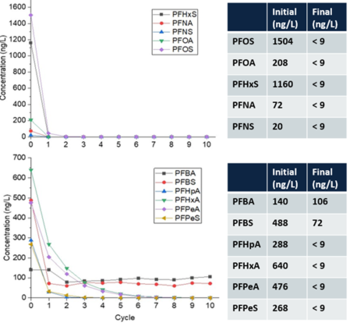
Over 300 gallons of PFAS-impacted groundwater were treated at a maximum flowrate of 1.1 gallons per minute (gpm) resulting in ≥90% reduction (mean percent removal of 99.7%) of long-chain PFAAs (fluorocarbon chain ≥ 6) and PFAS precursors in a single pass through the reactor (Figure 5) at a treatment cost of $7.30/1000 gallons[30]. As expected, the removal of short-chain PFAS was slower due to their lower potential for interfacial adsorption compared to long-chain PFAS. However, post-field laboratory studies revealed that the addition of a cationic surfactant such as CTAB (cetrimonium bromide) minimizes bulk liquid transport limitations for short-chain PFAS by electrostatically interacting with these compounds and transporting them to the plasma-liquid interface where they are degraded[28]. Both bench and pilot-scale EC plasma-based process have been extended for the treatment of PFAS in membrane concentrate, ion exchange brine, and landfill leachate[31][32].
As a part of a currently-funded ESTCP project (ESTCP ER20-5535)[33], the Clarkson University team with the support of GSI Environmental Inc. is evaluating the effectiveness of their plasma process in treating diluted aqueous film-forming foams (AFFFs) as well as the benefits of pre-oxidation of PFAS precursors in high concentration AFFF solutions in terms of post-oxidation plasma treatment time, destruction efficiency and cost.
Advantages and Limitations of the Technology for PFAS Treatment
Advantages:
- High removal rates of long-chain PFAS (C5-C8) due to the production of versatile reactive species
- Requires no chemical additions and produces no residual waste
- Total organic carbon (TOC) concentration and other non-surfactant co-contaminants do not influence the process efficiency
- The process is mobile and scalable
- Versatile: can be used in batch and continuous systems
Limitations:
- Limited removal of short-chain PFAS due to their inability to concentrate at plasma-liquid interfaces. Addition of surfactants such as CTAB improves their removal and degradation rates.
- Excessive foaming caused by bubbling argon gas through a solution containing high (>10 mg/L) concentrations of long-chain (surfactant) PFAS may interfere with the formation of plasma.
Summary
PFAS are susceptible to plasma treatment because the hydrophobic PFAS accumulates at the gas-liquid interface, exposing more of the PFAS to the plasma. Plasma-based treatment of PFAS contaminated water successfully degrades PFOA and PFOS to below the EPA health advisory level of 70 ppt and accomplishes the near complete destruction of other PFAS within a short treatment time. PFAS concentration reductions of ≥90% and post-treatment concentrations below laboratory detection levels are common for long chain PFAS and precursors. The lack of sensitivity of plasma to co-contaminants, coupled with high PFAS removal and defluorination efficiencies, makes plasma-based water treatment a promising technology for the remediation of PFAS-contaminated water. The plasma treatment process is currently developed for ex situ application and can also be integrated into a treatment train[34].
References
- ^ Sunka, P., Babický, V., Clupek, M., Lukes, P., Simek, M., Schmidt, J., and Cernak, M., 1999. Generation of Chemically Active Species by Electrical Discharges in Water. Plasma Sources Science and Technology, 8(2), pp. 258-265. DOI: 10.1088/0963-0252/8/2/006
- ^ Mededovic Thagard, S., Takashima, K., and Mizuno, A., 2009. Chemistry of the Positive and Negative Electrical Discharges Formed in Liquid Water and Above a Gas-Liquid Surface. Plasma Chemistry and Plasma Processing, 29(6), pp.455-473. DOI: 10.1007/s11090-009-9195-x
- ^ Du, C., Gong, X., and Lin, Y., 2019. Decomposition of volatile organic compounds using corona discharge plasma technology. Journal of the Air and Waste Management Association, 69(8), pp.879-899. DOI: 10.1080/10962247.2019.1582441 Article pdf
- ^ Xiong, Y., Zhang, Q., Wandell, R., Bresch, S., Wang, H., Locke, B.R. and Tang, Y., 2019. Synergistic 1,4-Dioxane Removal by Non-Thermal Plasma Followed by Biodegradation. Chemical Engineering Journal, 361, pp.519-527. DOI: 10.1016/J.CEJ.2018.12.094
- ^ Ni, G.H., Zhao, Y., Meng, Y.D., Wang, X.K., and Toyoda, H., 2013. Steam plasma jet for treatment of contaminated water with high-concentration 1,4-dioxane organic pollutants. Europhysics Letters, 101(4), p.45001. DOI: 10.1209/0295-5075/101/45001
- ^ Stratton, G.R., Bellona, C.L., Dai, F., Holsen, T.M. and Mededovic Thagard, S., 2015. Plasma-Based Water Treatment: Conception and Application of a New General Principle for Reactor Design. Chemical Engineering Journal, 273, pp.543-550. DOI: 10.1016/j.cej.2015.03.059
- ^ 7.0 7.1 7.2 Singh, R.K., Multari, N., Nau-Hix, C., Anderson, R.H., Richardson, S.D., Holsen, T.M. and Mededovic Thagard, S., 2019. Rapid Removal of Poly- and Perfluorinated Compounds from Investigation-Derived Waste (IDW) in a Pilot-Scale Plasma Reactor. Environmental Science and Technology, 53(19), pp.11375-11382. DOI: 10.1021/acs.est.9b02964
- ^ Singh, R.K., Fernando, S., Baygi, S.F., Multari, N., Mededovic Thagard, S., and Holsen, T.M., 2019. Breakdown Products from Perfluorinated Alkyl Substances (PFAS) Degradation in a Plasma-Based Water Treatment Process. Environmental Science and Technology, 53(5), pp.2731-2738. DOI: 10.1021/acs.est.8b07031
- ^ 9.0 9.1 Nzeribe, B.N., Crimi, M., Mededovic Thagard, S. and Holsen, T.M., 2019. Physico-Chemical Processes for the Treatment of Per- And Polyfluoroalkyl Substances (PFAS): A review. Critical Reviews in Environmental Science and Technology, 49(10), pp.866-915. DOI: 10.1080/10643389.2018.1542916
- ^ Laroussi, M., Bekeschus, S., Keidar, M., Bogaerts, A., Fridman, A., Lu, X.P., Ostrikov, K.K., Hori, M., Stapelmann, K., Miller, V., Reuter, S., Laux, C., Mesbah, A., Walsh, J., Jiang, C., Mededovic Thagard, S., Tanaka, H., Liu, D.W., Yan, D., and Yusupov, M., 2021. Low Temperature Plasma for Biology, Hygiene, and Medicine: Perspective and Roadmap. IEEE Transactions on Radiation and Plasma Medical Sciences. DOI: 10.1109/TRPMS.2021.3135118 Article pdf
- ^ Van Veldhuizen, E.M., and Rutgers, W.R., 2002. Pulsed Positive Corona Streamer Propagation and Branching. Journal of Physics D: Applied Physics, 35(17), p.2169. DOI: 10.1088/0022-3727/35/17/313
- ^ Yang, Y., Cho, Y.I. and Fridman, A., 2012. Plasma Discharge in Liquid: Water Treatment and Applications. CRC press. ISBN: 978-1-4398-6623-8 DOI: 10.1201/b11650
- ^ Rezaei, F., Vanraes, P., Nikiforov, A., Morent, R., and De Geyter, N., 2019. Applications of Plasma-Liquid Systems: A Review. Materials, 12(17), article 2751, 69 pp. DOI: 10.3390/ma12172751 Article pdf
- ^ Herianto, S., Hou, C.Y., Lin, C.M., and Chen, H.L., 2021. Nonthermal plasma-activated water: A comprehensive review of this new tool for enhanced food safety and quality. Comprehensive Reviews in Food Science and Food Safety, 20(1), pp. 583-626. DOI: 10.1111/1541-4337.12667
- ^ 15.0 15.1 Jiang, B., Zheng, J., Qiu, S., Wu, M., Zhang, Q., Yan, Z. and Xue, Q., 2014. Review on Electrical Discharge Plasma Technology for Wastewater Remediation. Chemical Engineering Journal, 236, pp. 348–368. DOI: 10.1016/j.cej.2013.09.090
- ^ Magureanu, M., Bradu, C., and Parvulescu, V.I., 2018. Plasma Processes for the Treatment of Water Contaminated with Harmful Organic Compounds. Journal of Physics D: Applied Physics, 51(31), p. 313002. DOI: 10.1088/1361-6463/aacd9c
- ^ Clements, J.S., Sato, M., and Davis, R.H., 1987. Preliminary Investigation of Prebreakdown Phenomena and Chemical Reactions Using a Pulsed High-Voltage Discharge in Water. IEEE Transactions on Industry Applications, IA-23(2), pp. 224-235. DOI: 10.1109/TIA.1987.4504897
- ^ Zeghioud, H., Nguyen-Tri, P., Khezami, L., Amrane, A., and Assadi, A.A., 2020. Review on Discharge Plasma for Water Treatment: Mechanism, Reactor Geometries, Active Species and Combined Processes. Journal of Water Process Engineering, 38, p.101664. DOI: 10.1016/j.jwpe.2020.101664
- ^ Murugesan, P., Evanjalin Monica, V., Moses, J.A., and Anandharamakrishnan, C., 2020. Water Decontamination Using Non-Thermal Plasma: Concepts, Applications, and Prospects. Journal of Environmental Chemical Engineering, 8(5), p. 104377. DOI: 10.1016/j.jece.2020.104377
- ^ Hayashi, R., Obo, H., Takeuchi, N., and Yasuoka, K., 2015. Decomposition of Perfluorinated Compounds in Water by DC Plasma within Oxygen Bubbles. Electrical Engineering in Japan, 190(3), pp.9-16. DOI: 10.1002/eej.22499 Article pdf.
- ^ Matsuya, Y., Takeuchi, N., Yasuoka, K., 2014. Relationship Between Reaction Rate of Perfluorocarboxylic Acid Decomposition at a Plasma-Liquid Interface and Adsorbed Amount. Electrical Engineering in Japan, 188(2), pp.1-8. DOI:10.1002/eej.22526 Article pdf.
- ^ Stratton, G.R., Dai, F., Bellona, C.L., Holsen, T.M., Dickenson, E.R., and Mededovic Thagard, S., 2017. Plasma-Based Water Treatment: Efficient Transformation of Perfluoroalkyl Substances in Prepared Solutions and Contaminated Groundwater. Environmental Science and Technology, 51(3), pp.1643-1648. DOI: 10.1021/acs.est.6b04215
- ^ Takeuchi, N., Kitagawa, Y., Kosugi, A., Tachibana, K., Obo, H., and Yasuoka, K., 2013. Plasma-Liquid Interfacial Reaction in Decomposition of Perfluoro Surfactants. Journal of Physics D: Applied Physics, 47(4), p.045203. DOI: 10.1088/0022-3727/47/4/045203
- ^ Yasuoka, K., Sasaki, K., and Hayashi, R., 2011. An Energy-Efficient Process for Decomposing Perfluorooctanoic and Perfluorooctane Sulfonic Acids Using DC Plasmas Generated within Gas Bubbles. Plasma Sources Science and Technology, 20(3), p. 034009. DOI:10.1088/0963-0252/20/3/034009
- ^ Yasuoka, K., Sasaki, K., Hayashi, R., Kosugi, A., and Takeuchi, N., 2010. Degradation of Perfluoro Compounds and F- Recovery in Water Using Discharge Plasmas Generated within Gas Bubbles. International Journal of Plasma Environmental Science and Technology, 4(2), 113–117. DOI:10.34343/ijpest.2010.04.02.113 Article pdf
- ^ Lewis, A.J., Joyce, T., Hadaya, M., Ebrahimi, F., Dragiev, I., Giardetti, N., Yang, J., Fridman, G., Rabinovich, A., Fridman, A.A., McKenzie, E.R., and Sales, C.M., 2020. Rapid Degradation of PFAS in Aqueous Solutions by Reverse Vortex Flow Gliding Arc Plasma. Environmental Science: Water Research and Technology, 6(4), pp.1044-1057. DOI: 10.1039/c9ew01050e
- ^ Saleem, M., Biondo, O., Sretenović, G., Tomei, G., Magarotto, M., Pavarin, D., Marotta, E. and Paradisi, C., 2020. Comparative Performance Assessment of Plasma Reactors for the Treatment of PFOA; Reactor Design, Kinetics, Mineralization and Energy Yield. Chemical Engineering Journal, 382, p.123031. DOI: 10.1016/j.cej.2019.123031
- ^ 28.0 28.1 Palma, D., Papagiannaki, D., Lai, M., Binetti, R., Sleiman, M., Minella, M. and Richard, C., 2021. PFAS Degradation in Ultrapure and Groundwater Using Non-Thermal Plasma. Molecules, 26(4), p. 924. DOI: 10.3390/molecules26040924 Article pdf
- ^ US Environmental Protection Agency (EPA), 2016. Lifetime Health Advisories and Health Effects Support Documents for Perfluorooctanoic Acid and Perfluorooctane Sulfonate. Federal Register, Notices, 81(101), p. 33250-33251. Register pdf.
- ^ 30.0 30.1 30.2 Nau-Hix, C., Multari, N., Singh, R.K., Richardson, S., Kulkarni, P., Anderson, R.H., Holsen, T.M. and Mededovic Thagard, S., 2021. Field Demonstration of a Pilot-Scale Plasma Reactor for the Rapid Removal of Poly-and Perfluoroalkyl Substances in Groundwater. ACS ES&T Water, 1(3), pp. 680-687. DOI: 10.1021/acsestwater.0c00170
- ^ Singh, R.K., Multari, N., Nau-Hix, C., Woodard, S., Nickelsen, M., Mededovic Thagard, S. and Holsen, T.M., 2020. Removal of Poly- And Per-Fluorinated Compounds from Ion Exchange Regenerant Still Bottom Samples in a Plasma Reactor. Environmental Science and Technology, 54(21), pp.13973-13980. DOI: 10.1021/acs.est.0c02158
- ^ Singh, R.K., Brown, E., Mededovic Thagard, S., and Holsen, T.M., 2021. Treatment of PFAS-Containing Landfill Leachate Using an Enhanced Contact Plasma Reactor. Journal of Hazardous Materials, 408, p.124452. DOI: 10.1016/j.jhazmat.2020.124452
- ^ Mededovic, S., 2020. An Innovative Plasma Technology for Treatment of AFFF Rinsate from Firefighting Delivery Systems. Environmental Security Technology Certification Program (ESTCP), Project ER20-5355.
- ^ Richardson, S., 2021. Nanofiltration Followed by Electrical Discharge Plasma for Destruction of PFAS and Co-occurring Chemicals in Groundwater: A Treatment Train Approach. Environmental Security Technology Certification Program (ESTCP), Project ER21-5136
See Also
SERDP and ESTCP PFAS Reserach and Remediation Podcast : Plasma Destruction of PFAS in Groundwater