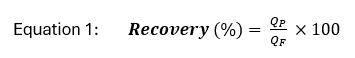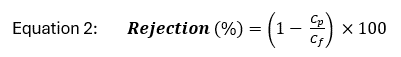User:Jhurley/sandbox
Reverse Osmosis and Nanofiltration Systems for PFAS Removal
Nanofiltration (NF) or reverse osmosis (RO) are engineered polymeric filters designed to remove solutes down to the atomic and molecular size scale[1][2][3]. RO, and to a lesser extent NF, has been implemented in a variety of water treatment applications including seawater and brackish water desalination, surface water treatment, industrial process water separation, and purification applications[1][2][3][4][5][6][7][8]. RO and NF use semi-permeable membranes that limit diffusion of solutes into the product water (i.e., permeate) through steric and electrostatic exclusion from the membrane polymer[2]. Due to the molecular size and ionic character of per- and polyfluoroalkyl substances (PFAS), past research has demonstrated that both RO and NF membranes can achieve a high degree of separation (i.e., rejection) of PFAS[9][10][11].
Contents
- 1 Reverse Osmosis and Nanofiltration Systems for PFAS Removal
- 2 Introduction
- 3 Application of High-Pressure Membranes for Treatment of PFAS Contaminated Water
- 4 Key Properties of PCM for MC Hydrolysis
- 5 Enhanced Hydrolysis of TNT and DNAN with Modified PCM
- 6 Computational Modeling
- 7 Exceptionally Strong NTO Adsorption on PCM
- 8 Performance of Modified PCM as Soil Amendments for IHE Post-Detonation Residues
- 9 References
- 10 See Also
Related Article(s):
- Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS)
- PFAS Ex Situ Water Treatment
- PFAS Sources
- PFAS Transport and Fate
Contributors: Christopher Bellona, Nicole Masters, Stephen Richardson
Key Resource(s):
- Interstate Technology Regulatory Council (ITRC), PFAS – Per- and Polyfluoroalkyl Substances: 12.2 Field-Implemented Liquids Treatment Technologies and 12.5 Limited Application and Developing Liquids Treatment Technologies
Introduction
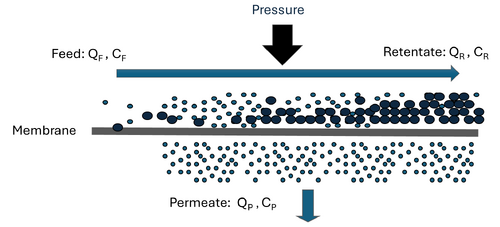
High-pressure membrane filtration such as nanofiltration (NF) or reverse osmosis (RO) is a filtration process that separates dissolved inorganic and organic solutes from liquid solvents, typically water[1]. As opposed to porous and more permeable low-pressure membranes (i.e., microfiltration and ultrafiltration), NF and RO membranes are widely considered semi-permeable and therefore require higher operating pressures to force water against an osmotic gradient to produce a purified permeate stream[2][3]. The semi-permeable nature and properties of RO and NF membranes results in significantly lower solute diffusive flux across the membranes compared to water[2].
To optimize solute separation and minimize accumulation of solutes on the membrane, these systems are almost exclusively operated in a cross-flow configuration where feed water flows parallel to the membrane surface and is forced across the membrane through the application of pressure (Figure 1). In a cross-flow configuration, NF and RO systems are separation processes that yield two streams: the treated permeate and the concentrated retentate.
Typical parameters used to describe operational performance of high-pressure membrane systems include solvent recovery and solute rejection. Recovery is defined as the percentage of feed water that becomes permeate, which can be calculated as:
where QP is the permeate flow rate, and QF is the feed flow rate. The recovery of a high-pressure membrane system is dependent upon the RO system configuration and feed water quality. For feed waters containing relatively low total dissolved solids (TDS) concentrations, in conventional RO and NF membrane applications, recovery is typically between 75% and 85%. However, several novel membrane configurations have been developed to increase membrane recoveries to 90% and greater depending on feed water quality.
Solute rejection is defined as the percent of concentrated feed water retained by the membrane and can be calculated as:
where Cp and Cf are the concentration of a solute in the permeate and feed water, respectively. Because the retentate stream contains high concentrations of all solutes rejected by the membrane, minimization of retentate volume is a focus of ongoing research and development[4][5].
Significant advancements in membrane material development have led to development of NF and RO membranes with varying pressure requirements and solute rejection characteristics[2][6]. RO utilizes very tight and selective membrane material (typically polyamide) that can achieve high rejection of most dissolved solutes but requires relatively high pressures, typically >150 psi depending on TDS concentration and RO membrane type (e.g., requiring up to 1000 psi when treating seawater with RO membrane elements optimized for seawater)[7]. RO is used in a variety of applications where a high degree of solute separation is desired including seawater and brackish water desalination, potable water reuse applications, industrial water treatment, and separation applications[1]. NF is fundamentally similar to RO; however, NF has been engineered to provide selective separation of solutes and often operate at lower pressures than RO (<150 psi). NF membranes have a range of rejection characteristics with some NF membranes being ‘tighter’ with lower permeability similar to RO (i.e., high salt and organic solute rejection) and others being ‘looser’ with high permeability (i.e., lower salt and organic solute rejection)[8].
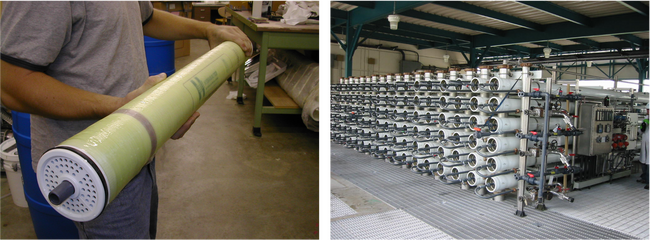
High-pressure NF and RO membranes are commonly found in a spiral-wound configuration[1]. Spiral-wound elements come in standardized sizes that are then loaded into a series of pressure vessels. An example of a spiral-wound element and a membrane system comprised of multiple pressure vessels is shown in Figure 2. Large-scale membrane systems are typically comprised of several membrane “stages” to increase recovery. Each stage contains multiple pressure vessels containing several individual spiral-wound elements each.
Application of High-Pressure Membranes for Treatment of PFAS Contaminated Water
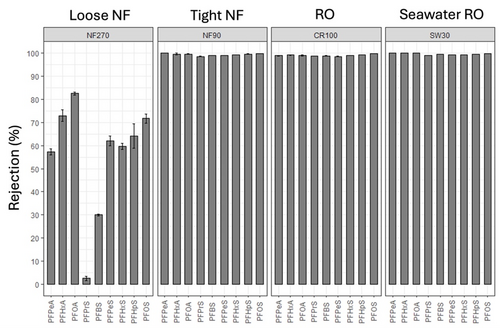
The effectiveness of RO and NF membranes for dissolved solute rejection has led to high-pressure membranes being regarded as one of the best available technologies for PFAS removal for over a decade[9][10]. Several studies have evaluated aspects of PFAS removal by NF and RO membranes including evaluating different membrane products, the impact of operating conditions and water quality, and the influence of physicochemical characteristics of PFAS[9][11][12][13][14][15][16]. Most studies have focused on anionic (at neutral pH) perfluoroalkyl acid (PFAA) rejection and reported greater than 90% separation of PFAAs by available NF and RO membranes due to electrostatic and steric exclusion from the membrane polymer[9][10][12]. Water quality constituents such as organic matter and cations including calcium and magnesium have been shown to reduce rejection of PFAS[12]. However, little is known about how fouling and membrane aging impact rejection of PFAS by NF and RO membranes and additional data are needed. A recent Department of Defense
Key Properties of PCM for MC Hydrolysis
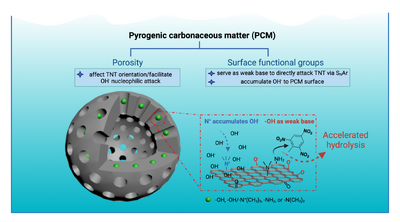
To investigate the effect of PCM functional group identity and pore characteristics on MC hydrolysis, a PCM-like polymer (PLP) platform was developed to allow for delineating individual PCM properties’ contributions using a reductionist approach. PLPs exhibit properties that are similar to PCM: (i) large surface area and high microporosity, (ii) highly conjugated and amorphous, and (iii) superior affinity for apolar organic contaminants. Unlike PCM, the attributes of PLP can be individually tuned and made homogeneously throughout the polymer networks. Specifically, six PLPs were synthesized via cross-coupling chemistry with specific functionality (i.e., -OH, -NH2, -N(CH3)2, and -N(CH3)3+) and pore characteristics (i.e., mesopore, micropore). Different surface functional groups were incorporated into the PLPs by adapting the synthesis approach described in previous work[17]. The pore characteristics of PLP were controlled by rigid node-strut topology, where two polymers were obtained, one with exclusively mesopores and the other with a mixture of micropores and mesopores. This showed that -OH and -NH2 functional groups can serve as weak bases to facilitate TNT hydrolysis, whereas -N(CH3)3+ groups can increase the local pH by accumulating OH- near PCM surfaces. Moreover, the micropores of PCM seem capable of altering the chemical environment around TNT molecules (including their location and orientation) in such a way as to facilitate their hydrolysis. This study attempted to scrutinize the complex properties of PCM that promote surface hydrolysis, namely the functional group identity and pore characteristics.
Enhanced Hydrolysis of TNT and DNAN with Modified PCM
This investigation proposes that PCMs can affect the thermodynamics and kinetics of hydrolysis reactions by confining the reaction species near PCM surfaces, thus making them less accessible to solvent molecules and creating an environment with a weaker dielectric constant that favors nucleophilic substitution reactions. The addition of QA groups on the PCM surface can further accelerate MC hydrolysis. The performance of PCM toward DNAN hydrolysis was evaluated by comparing the MC decay kinetics across various PCM types, including unmodified PCMs such as almond shell char or activated carbon (AC)), and modified PCMs with physical or chemically attached QA groups. The results suggest that QA-modified activated carbon performed the best by reducing the half-life of DNAN to 2.5 days at pH 11.5 and 25°C while maintaining its reactivity over ten consecutive additions of DNAN[18]. TNT exhibited faster decay in samples containing QA-modified AC than unmodified AC, with an estimated half-life of 0.2 days and 1 day, respectively[17]. Nitrite was observed as one of the transformation products for both DNAN and TNT, suggesting the presence of PCM favored the denitration pathway. By contrast, demethylation, the preferred pathway in homogeneous solution, produces 2,4-dinitrophenol (DNP). Denitration catalyzed by PCM was advantageous when compared to demethylation because nitrite is less toxic than DNAN and DNP. Overall, the results suggest that further improvement of the PCM performance could be expected by tailoring its surface to increase the abundance of QA while decreasing the presence of -NH2 or -OH groups for the hydrolysis of MCs.
Computational Modeling
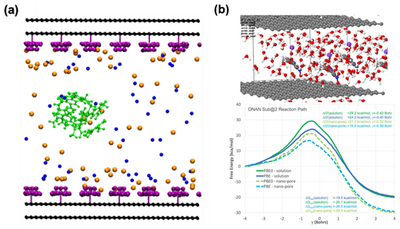
Further mechanistic insights were obtained by performing non-reactive molecular dynamics simulations on idealized pore structures. Upon the introduction of positively charged QA groups, the structure changed dramatically at the PCM interface. As the number of surface groups increased, the resulting density of OH- at the PCM surface increased by a factor of four relative to the density in the middle of the pore. Hence, the impact of the surface-bound cations was to attract OH- in competition with the neutralizing anions in the environment. In addition to driving the accumulation of OH-, the surface QA groups also impacted the distribution of TNT in the pore. At low QA surface coverage, TNT sought to adsorb on the exposed graphene. However, at sufficiently high QA surface coverage, TNT was blocked from lying flat on the graphene sheet and instead aggregated in the fluid away from the pore wall. The observation of TNT surface layering at intermediate charge densities was intriguing because it demonstrated the collection of TNT molecules close to the surface in the same spatial region where hydroxide was likewise accumulating relative to its concentration in the interstitial fluid. The molecular dynamics simulations provided evidence that the presence of the surface groups can play a role in accelerating TNT hydrolysis by acting to concentrate both TNT and hydroxide near the pore wall[17].
Ab Initio Molecular Dynamics/Molecular Mechanics (AIMD/MM) free energy simulations using expanded slabs and unit cells were also performed, focusing on the interaction of DNAN, hydroxide ions, Na+, and multiple water molecules sandwiched between two graphene layers[18]. The upper panel of Figure 3(b) provides a molecular snapshot from the AIMD/MM simulation, showcasing the intermediate stage of DNAN reacting with a hydroxide ion within a nano-pore structure. The lower panel depicts the reaction energy profiles for the hydrolysis of DNAN, both in bulk aqueous solution and within the nano-pore environment. The x-axis represents the reaction coordinate, a schematic representation of the progression from reactants to products through various transition states and intermediates. The y-axis corresponds to the Gibbs free energy changes (ΔG), providing insights into the thermodynamic favorability of each step in the pathway. Lower barriers corresponded to more kinetically accessible reactions. In the nano-pore environment, the energy barriers were significantly reduced, suggesting a catalytic effect due to confinement. This reduction was quantified by a decrease in ΔG of approximately 8 kcal/mol compared to the bulk solution, indicating that the reactions were not only more thermodynamically favorable but also kinetically accelerated in the nano-pore. In conclusion, the results demonstrate that nano-pore environments can significantly alter the hydrolysis mechanism of DNAN, leading to potentially less toxic products.
Exceptionally Strong NTO Adsorption on PCM
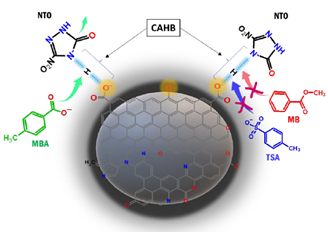
Results from this project suggest that NTO was chemically stable for up to at least a week in NaOH solution at pH 13.8[19]. Despite its highly polar and anionic character (pKa = 3.78), NTO exhibited unexpectedly strong sorption toward PCM at environmentally relevant pH conditions. This high affinity was partly due to the formation of an exceptionally strong negative charge-assisted hydrogen bond, or (−)CAHB, with weak acid functional groups on the carbon surface. The CAHB was identified by evaluating adsorption isotherms, pH adsorption edge plots, competitive sorption experiments, and pH drift experiments. The findings contradict the conventional view that polar organic anions have little affinity for or are even repelled by hydrophobic carbonaceous sorbents. The results call attention to the need for new models or modification of existing models for the sorption of ionizable compounds that consider CAHB formation with sorbents. The findings also have potential implications for the use of carbons in environmental remediation and catalysis, particularly for the design of strategies for the retention and degradation of highly mobile contaminants.
Performance of Modified PCM as Soil Amendments for IHE Post-Detonation Residues
Batch and column tests were conducted to evaluate the adsorption and hydrolysis of post-detonation residues of IMX-101 in three DoD range soils amended with modified PCMs[20]. Results indicated that adding PCMs enhanced the removal of NTO, NQ, and DNAN in soils compared to the soil controls, with enhancement factors ranging from 50 to 300. Consistent with previous results, NTO exhibited the highest partition coefficients (Kd) in PCM-amended soils compared to DNAN and NQ despite its highly polar and anionic character. Among various PCMs, QA-modified AC performed best, followed by unmodified AC and chars. The Kd values of NTO, NQ, and DNAN were slightly lower in the IMX-101 mixture than individually, possibly due to the adsorption competition from other constituents in IMX-101. The treatment was evaluated at pH 8, 10, and 12. No NTO decay was observed across the investigated pH range with or without PCM. By contrast, up to 13% of NQ was removed but only at pH > 10. Up to 90% DNAN decay occurred at pH 10 and 12 over 7 days in soils amended with modified AC. The 2% amendment dose was most effective, maintaining its adsorption capacity and reactivity over three consecutive IMX-101 additions. Column tests confirmed that 2% PCM addition significantly delayed the NTO, NQ, and DNAN breakthrough. The breakthrough volume (defined as treatment volume resulting in Concentrationout=0.1*Concentrationin) of NTO, NQ, and DNAN correlated with their Kd values obtained from the batch tests, where no retention was observed in the absence of PCM amendments. These findings highlight the feasibility of using modified PCM to simultaneously retain and transform IMX residues, providing a strategy for using reactive amendments in situ to sustain military operation and pollutant abatement.
References
- ^ 1.0 1.1 1.2 1.3 1.4 Wilf, M., 2019. Basic Terms and Definitions, Chapter 3 in Desalination: Water from Water, 2nd Edition, J. Kucera, Editor. John Wiley & Sons. ISBN: 978-1-119-40774-4 doi: 10.1002/9781119407874.ch3
- ^ 2.0 2.1 2.2 2.3 2.4 2.5 Bellona, C., Drewes, J., Xu, P., Amy, G., 2004. Factors affecting the rejection of organic solutes during NF/RO treatment—a literature review. Water Research, 38(12), p. 2795-2809. doi: 10.1016/j.watres.2004.03.034
- ^ 3.0 3.1 3.2 Bazargan, A., Salgado, B., 2018. Fundamentals of Desalination Technology, in A Multidisciplinary Introduction to Desalination, A. Bazargan, Editor. River Publishers. p. 41-66. ISBN 9788793379541. doi: 10.1201/9781003336914
- ^ 4.0 4.1 Turek, M., Mitko, K., Piotrowski, K., Dydo, P., Laskowska, E., Jakóbik-Kolon, A., 2017. Prospects for high water recovery membrane desalination. Desalination, 401, p. 180-189. doi: 10.1016/j.desal.2016.07.047
- ^ 5.0 5.1 Panagopoulos, A., Haralambous, K.-J., Loizidou, M., 2019. Desalination brine disposal methods and treatment technologies - A review. Science of The Total Environment, 693, Article 133545. doi: 10.1016/j.scitotenv.2019.07.351
- ^ 6.0 6.1 Warsinger, D.M., Chakraborty, S., Tow, E.W., Plumlee, M.H., Bellona, C., Loutatidou, S., Karimi, L., Mikelonis, A.M., Achilli, A., Ghassemi, A., Padhye, L.P., Snyder, S.A., Curcio, S., Vecitis, C.D., Arafat, H.A., Lienhard, J.H., 2018. A review of polymeric membranes and processes for potable water reuse. Progress in Polymer Science, 81, p. 209-237. doi: 10.1016/j.progpolymsci.2018.01.004
- ^ 7.0 7.1 Yan, D., 2017. Membrane Desalination Technologies, Chapter 6 in A Multidisciplinary Introduction to Desalination, A. Bazargan, Editor. River Publishers, p. 155-199. ISBN: 9788793379541
- ^ 8.0 8.1 Bellona, C., 2019. Nanofiltration - Theory and Application, Chapter 4 in Desalination: Water from Water, 2nd Edition, J. Kucera, Editor. John Wiley & Sons. ISBN: 978-1-119-40774-4. doi: 10.1002/9781118904855.ch4
- ^ 9.0 9.1 9.2 9.3 Appleman, T.D., Dickenson, E.R.V., Bellona, C., Higgins, C.P., 2013. Nanofiltration and granular activated carbon treatment of perfluoroalkyl acids. Journal of Hazardous Materials, 260, p. 740-746. doi: 10.1016/j.jhazmat.2013.06.033
- ^ 10.0 10.1 10.2 Steinle-Darling, E., Reinhard, M., 2008. Nanofiltration for Trace Organic Contaminant Removal: Structure, Solution, and Membrane Fouling Effects on the Rejection of Perfluorochemicals. Environmental Science and Technology, 42(14), p. 5292-5297. doi: 10.1021/es703207s
- ^ 11.0 11.1 Safulko, A., Cath, T.Y., Li, F., Tajdini, B., Boyd, M., Huehmer, R.P., Bellona, C., 2023. Rejection of perfluoroalkyl acids by nanofiltration and reverse osmosis in a high-recovery closed-circuit membrane filtration system. Separation and Purification Technology, 326, Article 124867. doi: 10.1016/j.seppur.2023.124867 Open Access Manuscript
- ^ 12.0 12.1 12.2 Liu, C.J., Strathmann, T.J., Bellona, C., 2021. Rejection of per- and polyfluoroalkyl substances (PFASs) in aqueous film-forming foam by high-pressure membranes. Water Research, 188, Article 116546. doi: 10.1016/j.watres.2020.116546
- ^ Wang, J., Wang, L., Xu, C., Zhi, R., Miao, R., Liang, T., Yue, X., Lv, Y., Liu, T., 2018. Perfluorooctane sulfonate and perfluorobutane sulfonate removal from water by nanofiltration membrane: The roles of solute concentration, ionic strength, and macromolecular organic foulants. Chemical Engineering Journal, 332, p. 787-797. doi: 10.1016/j.cej.2017.09.061
- ^ Zhao, C., Tang, C.Y., Li, P., Adrian, P., Hu, G., 2016. Perfluorooctane sulfonate removal by nanofiltration membrane—the effect and interaction of magnesium ion / humic acid. Journal of Membrane Science, 503, p. 31-41. doi: 10.1016/j.memsci.2015.12.049
- ^ Zhao, C., Zhang, J., He, G., Wang, T., Hou, D., Luan, Z., 2013. Perfluorooctane sulfonate removal by nanofiltration membrane the role of calcium ions. Chemical Engineering Journal, 233, p. 224-232. doi: 10.1016/j.cej.2013.08.027
- ^ Steinle-Darling, E., Litwiller, E., Reinhard, M., 2010. Effects of Sorption on the Rejection of Trace Organic Contaminants During Nanofiltration. Environmental Science and Technology, 44(7), p. 2592-2598. doi: 10.1021/es902846m
- ^ 17.0 17.1 17.2 17.3 17.4 Li, Z., Jorn, R., Samonte, P.R.V., Mao, J., Sivey, J.D., Pignatello, J.J., Xu, W., 2022. Surface-catalyzed hydrolysis by pyrogenic carbonaceous matter and model polymers: An experimental and computational study on functional group and pore characteristics. Applied Catalysis B: Environment and Energy, 319, article 121877. doi: 10.1016/j.apcatb.2022.121877 Open Access Manuscript
- ^ 18.0 18.1 18.2 Cite error: Invalid
<ref>tag; no text was provided for refs namedSeenthiaEtAl2024 - ^ 19.0 19.1 Abdelraheem, W., Meng, L., Pignatello, J.J., Seenthia, N.I., Xu, W., 2024. Participation of Strong H-Bonding to Acidic Groups Contributes to the Intense Sorption of the Anionic Munition, Nitrotriazolone (NTO) to the Carbon, Filtrasorb 400. Environmental Science and Technology, 58(46), pp. 20719-20728. doi: 10.1021/acs.est.4c07055
- ^ Seenthia, N.I., Abdelraheem, W., Beal, S.A., Pignatello, J.J., Xu, W., 2025. Simultaneous adsorption and hydrolysis of insensitive munition compounds by pyrogenic carbonaceous matter (PCM) and functionalized PCM in soils. Journal of Hazardous Materials, 494, article 138501. doi: 10.1016/j.jhazmat.2025.138501