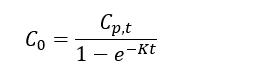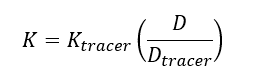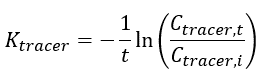User:Jhurley/sandbox
Hydrothermal Alkaline Treatment (HALT)
Hydrothermal alkaline treatment (HALT) is a thermochemical processing technology effective at destroying and defluorinating halogenated organic compounds such as per- and polyfluoroalkyl substances (PFAS). HALT is highly effective at destroying and defluorinating all types of PFAS that have been evaluated. The HALT technology enables end-to-end treatment and destruction of PFAS from a variety of matrices when integrated into a suitable treatment train.
Related Article(s):
- Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS)
- PFAS Ex Situ Water Treatment
- PFAS Sources
- PFAS Transport and Fate
Contributors:
- Dr. Brian Pinkard
- Dr. Timothy Strathmann
- Dr. Shilai Hao
Key Resource(s):
- Hydrothermal Technologies for On-Site Destruction of Site Investigation Wastes Impacted by PFAS[1]
- Hydrothermal Alkaline Treatment for Destruction of Per- and Polyfluoroalkyl Substances in Aqueous Film-Forming Foam[2]
- Degradation and Defluorination of Ultra Short-, Short-, and Long-Chain PFASs in High Total Dissolved Solids Solutions by Hydrothermal Alkaline Treatment ─ Closing the Fluorine Mass Balance[3]
Introduction
Hydrothermal alkaline treatment (HALT) is a thermochemical processing technology effective at destroying and defluorinating halogenated organic compounds such as per- and polyfluoroalkyl substances (PFAS). HALT is also known as “ alkaline hydrolysis,” and is very similar to processing technologies such as hydrothermal liquefaction (HTL) which have been developed and investigated for organic waste-to-energy applications.
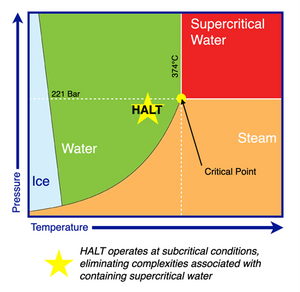
HALT processing subjects PFAS in an aqueous solution to high pressure, high temperature, and high pH conditions. The required operating conditions are dependent on the specific target PFAS being destroyed, as perfluorocarboxylic acids (PFCAs) such as trifluoroacetic acid (TFA) can be destroyed under mild conditions (e.g., P ~ 2 MPa, T ~ 200 °C, pH ~ 13)[4], whereas perfluorosulfonic acids (PFSAs) such as perfluorobutanesulfonic acid (PFBS) require more aggressive processing conditions (e.g., P ~ 25 MPa, T ~ 350 °C, pH ~ 14.7) [5] (Figure 1) . HALT is capable of facilitating complete “mineralization” of PFAS, defined as the conversion of organic fluorine to dissolved inorganic fluoride. The treatment time for HALT is relatively shorter (<2 hours) compared to most other PFAS destructive technologies. For instance, treatment of two-fold diluted aqueous film-forming foams (AFFFs) using HALT in batch mode achieved nearly complete defluorination in just 30 minutes under conditions of 350 °C and 5 M NaOH[2]. PFCAs can be destroyed with even faster kinetics at milder conditions; for example, >90% destruction and defluorination of trifluoroacetic acid (TFA) was achieved within 40 min at 200 °C[4]. Kinetic rate constants for individual PFAS compounds in HALT environments have been proposed in several studies[4][5]. Several studies have also investigated the fluorine mass balance during HALT processing, showing near-stoichiometric conversion of organic fluorine to inorganic fluoride under optimal conditions[3].
From a practical perspective, HALT is best suited for destroying PFAS in concentrated liquids such as liquid concentrate streams produced as byproducts of other water treatment processes (e.g., regenerable ion exchange, foam fractionation). Previous publications demonstrate that complex sample matrices, including high concentrations of inorganic salts (e.g., 83 g/L chloride) and dissolved organic carbon (e.g., 13 g/L), do not inhibit the degradation rate of PFAS compared to a clean matrix, such as groundwater[6][7]. Moreover, several field demonstrations of HALT have been performed successfully, and the technology is scalable for commercialization.
Reaction Mechanisms and Treatment Efficacy
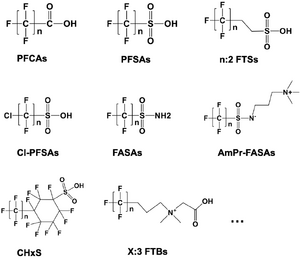
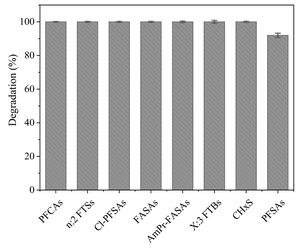
Laboratory scale batch experiments have shown that the full suite of PFAS detected in aqueous film-forming foams (AFFFs) through targeted LC-MS/MS and LC-HRMS suspect screening analysis are degraded and defluorinated by HALT[2]. Figure 2 presents representative classes of PFAS structures among 148 PFAS demonstrating complete degradation during HALT. Figure 3 illustrates the degradation during HALT of representative classes of PFAS detected in an AFFF. The extent of destruction for all PFAS is highly temperature dependent, but results show that some subclasses of PFAS degrade in the absence of alkali amendments (e.g., PFCAs)[4], whereas other subclasses require strong alkali in addition to near-critical reaction temperatures (e.g., PFSAs)[1][2][5]. This is attributed to different mechanisms that initiate the destruction of the individual PFAS subclasses. Degradation of PFCAs is initiated by thermally driven decarboxylation reactions[4], whereas PFSA degradation, in the temperature range of HALT reactors, is proposed to be initiated via attack by the strong nucleophile OH-.[2]
A mechanistic understanding of the HALT process for PFAS destruction needs further evaluation to optimize the process and reduce the consumption of chemicals and energy. While the studies of neat compounds are relatively straightforward, one of the major challenges is to address the effect of co-contaminants and apply the process to real-world operating scenarios. Recent laboratory studies with batch reactors conducted at the Colorado School of Mines (CSM) have extended the application of HALT for the destruction of PFAS in a variety of contaminated matrices, including groundwater and soils[6] and foam fractionation-derived liquid concentrate[7]. Apparent rates for the transformation of individual PFAS have been found to be largely insensitive to the type of media[7], but there is a need to account for any reactions with the media that consume OH· (e.g., OH· reactions with silica-containing soil minerals)[6] Notably, while alkali is not required to degrade PFCAs, it is still necessary to convert the organically bound fluorine to inorganic F-. Austin et. al.[4] showed that TFA, a C1 PFCA, degrades at similar rates in the absence and presence of NaOH, but mineralization to F- and CO32- only occurs when NaOH is added; otherwise fluoroform (CHF3) is the terminal product when no NaOH is added to the reaction solution.
HALT can also be applied to destroy other fluorinated compounds, for example, hydrofluorocarbon (HFC) refrigerants. HFC refrigerants are known to decompose into PFAS such as TFA in the atmosphere and thereby subsequently appear in concerning concentrations in rainwater. By themselves, HFCs are resistant to thermal degradation; however, in the presence of alkali (e.g., NaOH), alkaline hydrolysis can occur at T < 150˚C[4].
Peepers Preparation, Deployment and Retrieval
Peepers are often prepared in laboratories but are also commercially available in a variety of designs from several suppliers. Peepers are prepared by first cleaning all materials to remove even trace levels of metals before assembly. The water contained inside the peeper is sometimes deoxygenated, and in some cases the peeper is maintained in a deoxygenated atmosphere until deployment[8]. However, recent studies[9] have shown that deoxygenation prior to deployment does not significantly impact sampling results due to oxygen rapidly diffusing out of the peeper during deployment. Once assembled, peepers are usually shipped in a protective bag inside a hard-case cooler for protection.
Peepers are deployed by insertion into sediment for a period of a few days to a few weeks. Insertion into the sediment can be achieved by wading to the location when the water depth is shallow, by using push poles for deeper deployments[9], or by professional divers for the deepest sites. If divers are used, an appropriate boat or ship will be required to accommodate the diver and their equipment. Whichever method is used, peepers should be attached to an anchor or a small buoy to facilitate retrieval at the end of the deployment period.
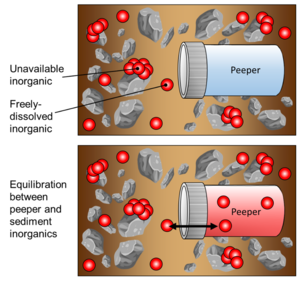
During deployment, passive sampling is achieved via diffusion of inorganics through the peeper’s semi-permeable membrane, as the enclosed volume of peeper water equilibrates with the surrounding sediment porewater (Figure 4). It is assumed that the peeper insertion does not greatly alter geochemical conditions that affect freely-dissolved inorganics. Additionally, it is assumed that the peeper water equilibrates with freely-dissolved inorganics in sediment in such a way that the concentration of inorganics in the peeper water would be equal to that of the concentration of inorganics in the sediment porewater.
After retrieval, the peepers are brought to the surface and usually preserved until they can be processed. This can be achieved by storing the peepers inside a sealable, airtight bag with either inert gas or oxygen absorbing packets[9]. The peeper water can then be processed by quickly pipetting it into an appropriate sample bottle which usually contains a preservative (e.g., nitric acid for metals). This step is generally conducted in the field. Samples are stored on ice to maintain a temperature of less than 4°C and shipped to an analytical laboratory. The samples are then analyzed for inorganics by standard methods (i.e., USEPA SW-846). The results obtained from the analytical laboratory are then used directly or assessed using the equations below if a reverse tracer is used because deployment time is insufficient for all analytes to reach equilibrium.
Equilibrium Determination (Tracers)
The equilibration period of peepers can last several weeks and depends on deployment conditions, analyte of interest, and peeper design. In many cases, it is advantageous to use pre-equilibrium methods that can use measurements in peepers deployed for shorter periods to predict concentrations at equilibrium[10].
Although the equilibrium concentration of an analyte in sediment can be evaluated by examining analyte results for peepers deployed for several different amounts of time (i.e., a time series), this is impractical for typical field investigations because it would require several mobilizations to the site to retrieve samplers. Alternately, reverse tracers (referred to as a performance reference compound when used with organic compound passive sampling) can be used to evaluate the percentage of equilibrium reached by a passive sampler.
Thomas and Arthur[11] studied the use of a reverse tracer to estimate percent equilibrium in lab experiments and a field application. They concluded that bromide can be used to estimate concentrations in porewater using measurements obtained before equilibrium is reached. Further studies were also conducted by Risacher et al.[9] showed that lithium can also be used as a tracer for brackish and saline environments. Both studies included a mathematical model for estimating concentrations of ions in external media (C0) based on measured concentrations in the peeper chamber (Cp,t), the elimination rate of the target analyte (K) and the deployment time (t):
The elimination rate of the target analyte (K) is calculated using Equation 2:
The elimination rate of the tracer (Ktracer) is calculated using Equation 3:
Using this set of equations allows the calculation of the porewater concentration of the analyte prior to its equilibrium with the peeper water. A template for these calculations can be found in the appendix of Risacher et al.[9].
Using Peeper Data at a Sediment Site
Peeper data can be used to enable site specific decision making in a variety of ways. Some of the most common uses for peepers and peeper data are discussed below.
Nature and Extent: Multiple peepers deployed in sediment can help delineate areas of increased metal availability. Peepers are especially helpful for sites that are comprised of coarse, relatively inert materials that may not be conducive to traditional bulk sediment sampling. Because much of the inorganics present in these types of sediments may be associated with the porewater phase rather than the solid phase, peepers can provide a more representative measurement of C0. Additionally, at sites where tidal pumping or groundwater flux may be influencing the nature and extent of inorganics, peepers can provide a distinct advantage to bulk sediment sampling or other point-in-time measurements, as peepers can provide an average measurement that integrates the variability in the hydrodynamic and chemical conditions over time.
Sources and Fate: A considerable advantage to using peepers is that C0 results are expressed as concentration in units of mass per volume (e.g., mg/L), providing a common unit of measurement to compare across multiple media. For example, synchronous measurements of C0 using peepers deployed in both surface water and sediment can elucidate the potential flux of inorganics from sediment to surface water. Paired measurements of both C0 and bulk metals in sediment can also allow site specific sediment-porewater partition coefficients to be calculated. These values can be useful in understanding and predicting contaminant fate, especially in situations where the potential dissolution of metals from sediment are critical to predict, such as when sediment is dredged.
Direct Toxicity to Aquatic Life: Peepers are frequently used to understand the potential direct toxicity to aquatic life, such as benthic invertebrates and fish. A C0 measurement obtained from a peeper deployed in sediment (in situ) or surface water (ex situ), can be compared to toxicological benchmarks for aquatic life to understand the potential toxicity to aquatic life and to set remediation goals[10]. C0 measurements can also be incorporated in more sophisticated approaches, such as the Biotic Ligand Model[12] to understand the potential for toxicity or the need to conduct toxicological testing or ecological evaluations.
Bioaccumulation of Inorganics by Aquatic Life: Peepers can also be used to understand site specific relationship between C0 and concentrations of inorganics in aquatic life. For example, measuring C0 in sediment from which organisms are collected and analyzed can enable the estimation of a site-specific uptake factor. This C0-to-organism uptake factor (or model) can then be applied for a variety of uses, including predicting the concentration of inorganics in other organisms, or estimating a sediment C0 value that would be safe for consumption by wildlife or humans. Because several decades of research have found that the correlation between C0 measurements and bioavailability is usually better than the correlation between measurements of chemicals in bulk sediment and bioavailability, C0-to-organism uptake factors are likely to be more accurate than uptake factors based on bulk sediment testing.
Evaluating Sediment Remediation Efficacy: Passive sampling has been used widely to evaluate the efficacy of remedial actions such as active amendments, thin layer placements, and capping to reduce the availability of contaminants at sediment sites. A particularly powerful approach is to compare baseline (pre-remedy) C0 in sediment to C0 in sediment after the sediment remedy has been applied. Peepers can be used in this context for inorganics, allowing the sediment remedy’s success to be evaluated and monitored in laboratory benchtop remedy evaluations, pilot scale remedy evaluations, and full-scale remediation monitoring.
References
- ^ 1.0 1.1 Strathmann, T.J., 2023. Hydrothermal Technologies for On-Site Destruction of Site Investigation Wastes Impacted by PFAS. Project number ER18-1501, Strategic Environmental Research and Development Program (SERDP).
- ^ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 Hao, S., Choi, Y.J., Wu, B., Higgins, C.P., Deeb, R., Strathmann, T.J., 2021. Hydrothermal Alkaline Treatment for Destruction of Per- and Polyfluoroalkyl Substances in Aqueous Film-Forming Foam. Environmental Science and Technology, 55(5), pp. 3283-3295. doi: 10.1021/acs.est.0c06906
- ^ 3.0 3.1 Pinkard, B., Smith, S.M., Vorarath, P., Smrz, T., Schmick, S., Dressel, L., Bryan, C., Czerski, M., de Marne, A., Halevi, A., Thomsen, C., Woodruff, C., 2024. Degradation and Defluorination of Ultra Short-, Short-, and Long-Chain PFASs in High Total Dissolved Solids Solutions by Hydrothermal Alkaline Treatment─Closing the Fluorine Mass Balance. ACS ES&T Engineering, 4(11), pp. 2810-2818. doi: 10.1021/acsestengg.4c00378 Open Access Report.pdf
- ^ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 Austin, C., Purohit, A., Thomsen, C., Pinkard, B.R., Strathmann, T.J., Novosselov, I.V., 2024. Hydrothermal Destruction and Defluorination of Trifluoroacetic Acid (TFA). Environmental Science and Technology, 58(18), pp. 8076-8085. doi: 10.1021/acs.est.3c09404
- ^ 5.0 5.1 Wu, B., Hao, S., Choi, Y.J., Higgins, C.P., Deeb, R., Strathmann, T.J., 2019. Rapid Destruction and Defluorination of Perfluorooctanesulfonate by Alkaline Hydrothermal Reaction. Environmental Science and Technology Letters, 6(10), pp. 630-636. doi: 10.1021/acs.estlett.9b00506
- ^ 6.0 6.1 6.2 Hao, S., Choi, Y.J,. Deeb, R.A., Strathmann, T.J., Higgins, C.P., 2022. Application of Hydrothermal Alkaline Treatment for Destruction of Per- and Polyfluoroalkyl Substances in Contaminated Groundwater and Soil. Environmental Science and Technology, 56(10), pp. 6647-6657. doi: 10.1021/acs.est.2c00654
- ^ 7.0 7.1 7.2 Hao, S., Reardon, P.N., Choi, Y.J., Zhang, C., Sanchez, J.M., Higgins, C.P., Strathmann, T.J., 2023. Hydrothermal Alkaline Treatment (HALT) of Foam Fractionation Concentrate Derived from PFAS-Contaminated Groundwater. Environmental Science and Technology 57(44), pp. 17154-17165. doi: 10.1021/acs.est.3c05140
- ^ Carignan, R., St‐Pierre, S., Gachter, R., 1994. Use of diffusion samplers in oligotrophic lake sediments: Effects of free oxygen in sampler material. Limnology and Oceanography, 39(2), pp. 468-474. doi: 10.4319/lo.1994.39.2.0468 Open Access Article
- ^ 9.0 9.1 9.2 9.3 9.4 Cite error: Invalid
<ref>tag; no text was provided for refs namedRisacherEtAl2023 - ^ 10.0 10.1 USEPA, 2017. Laboratory, Field, and Analytical Procedures for Using Passive Sampling in the Evaluation of Contaminated Sediments: User’s Manual. EPA/600/R-16/357. Report.pdf
- ^ Cite error: Invalid
<ref>tag; no text was provided for refs namedThomasArthur2010 - ^ Santore, C.R., Toll, E.J., DeForest, K.D., Croteau, K., Baldwin, A., Bergquist, B., McPeek, K., Tobiason, K., and Judd, L.N., 2022. Refining our understanding of metal bioavailability in sediments using information from porewater: Application of a multi-metal BLM as an extension of the Equilibrium Partitioning Sediment Benchmarks. Integrated Environmental Assessment and Management, 18(5), pp. 1335–1347. doi: 10.1002/ieam.4572