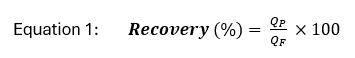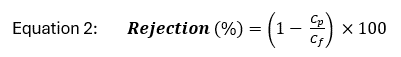Difference between revisions of "User:Jhurley/sandbox"
(→PFAS Toxicology and Risk Assessment) |
(→PFAS Toxicology and Risk Assessment) |
||
| Line 12: | Line 12: | ||
'''Key Resource(s):''' | '''Key Resource(s):''' | ||
| − | + | *State of the Science for Risk Assessment of PFAS at Contaminated Sites<ref name="ZodrowEtAl2021">Zodrow, J., Arblaster, J., Conder, J., 2021. “State of the Science for Risk Assessment of PFAS at Contaminated Sites.” In: Forever Chemicals: Environmental, Economic, and Social Equity Concerns with PFAS in the Environment, edited by Kempisty, D., Racz, L., pp. 161-186. CRC Press. [https://doi.org/10.1201/9781003024521 doi: 10.1201/9781003024521]</ref> | |
*[https://itrcweb.org/ Interstate Technology Regulatory Council (ITRC)], [https://pfas-1.itrcweb.org/ PFAS – Per- and Polyfluoroalkyl Substances] | *[https://itrcweb.org/ Interstate Technology Regulatory Council (ITRC)], [https://pfas-1.itrcweb.org/ PFAS – Per- and Polyfluoroalkyl Substances] | ||
Revision as of 14:25, 29 August 2025
PFAS Toxicology and Risk Assessment
Overview of current practices for human health and ecological risk assessment related to per- and poly-fluoroalkyl substances (PFAS) exposures at aqueous film-forming foam (AFFF) impacted sites.
Related Article(s):
Contributors: Jennifer Arblaster, Jason Conder, Jean Zodrow and Elizabeth Nichols
Key Resource(s):
- State of the Science for Risk Assessment of PFAS at Contaminated Sites[1]
- Interstate Technology Regulatory Council (ITRC), PFAS – Per- and Polyfluoroalkyl Substances
Introduction
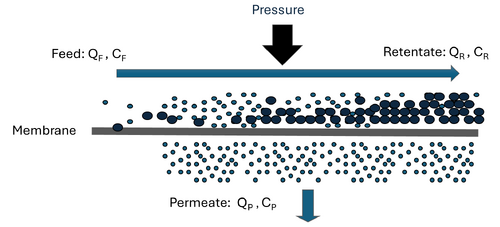
High-pressure membrane filtration such as nanofiltration (NF) or reverse osmosis (RO) is a filtration process that separates dissolved inorganic and organic solutes from liquid solvents, typically water[2]. As opposed to porous and more permeable low-pressure membranes (i.e., microfiltration and ultrafiltration), NF and RO membranes are widely considered semi-permeable and therefore require higher operating pressures to force water against an osmotic gradient to produce a purified permeate stream[3][4]. The semi-permeable nature and properties of RO and NF membranes results in significantly lower solute diffusive flux across the membranes compared to water[3].
To optimize solute separation and minimize accumulation of solutes on the membrane, these systems are almost exclusively operated in a cross-flow configuration where feed water flows parallel to the membrane surface and is forced across the membrane through the application of pressure (Figure 1). In a cross-flow configuration, NF and RO systems are separation processes that yield two streams: the treated permeate and the concentrated retentate.
Typical parameters used to describe operational performance of high-pressure membrane systems include solvent recovery and solute rejection. Recovery is defined as the percentage of feed water that becomes permeate, which can be calculated as:
where QP is the permeate flow rate, and QF is the feed flow rate. The recovery of a high-pressure membrane system is dependent upon the RO system configuration and feed water quality. For feed waters containing relatively low total dissolved solids (TDS) concentrations, in conventional RO and NF membrane applications, recovery is typically between 75% and 85%. However, several novel membrane configurations have been developed to increase membrane recoveries to 90% and greater depending on feed water quality.
Solute rejection is defined as the percent of concentrated feed water retained by the membrane and can be calculated as:
where Cp and Cf are the concentration of a solute in the permeate and feed water, respectively. Because the retentate stream contains high concentrations of all solutes rejected by the membrane, minimization of retentate volume is a focus of ongoing research and development[5][6].
Significant advancements in membrane material development have led to development of NF and RO membranes with varying pressure requirements and solute rejection characteristics[3][7]. RO utilizes very tight and selective membrane material (typically polyamide) that can achieve high rejection of most dissolved solutes but requires relatively high pressures, typically >150 psi depending on TDS concentration and RO membrane type (e.g., requiring up to 1000 psi when treating seawater with RO membrane elements optimized for seawater)[8]. RO is used in a variety of applications where a high degree of solute separation is desired including seawater and brackish water desalination, potable water reuse applications, industrial water treatment, and separation applications[2]. NF is fundamentally similar to RO; however, NF has been engineered to provide selective separation of solutes and often operate at lower pressures than RO (<150 psi). NF membranes have a range of rejection characteristics with some NF membranes being ‘tighter’ with lower permeability similar to RO (i.e., high salt and organic solute rejection) and others being ‘looser’ with high permeability (i.e., lower salt and organic solute rejection)[9].
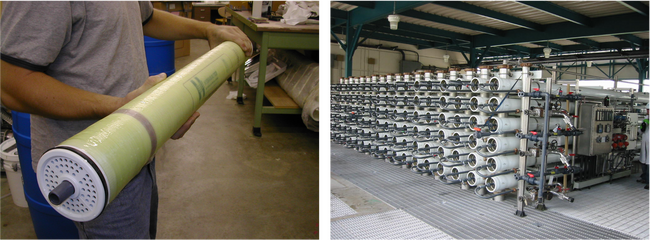
High-pressure NF and RO membranes are commonly found in a spiral-wound configuration[2]. Spiral-wound elements come in standardized sizes that are then loaded into a series of pressure vessels. An example of a spiral-wound element and a membrane system comprised of multiple pressure vessels is shown in Figure 2. Large-scale membrane systems are typically comprised of several membrane “stages” to increase recovery. Each stage contains multiple pressure vessels containing several individual spiral-wound elements each.
Application of High-Pressure Membranes for Treatment of PFAS Contaminated Water
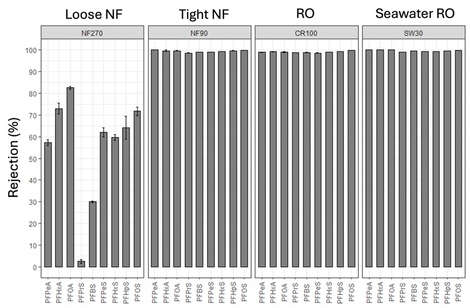
The effectiveness of RO and NF membranes for dissolved solute rejection has led to high-pressure membranes being regarded as one of the best available technologies for PFAS removal for over a decade[10][11]. Several studies have evaluated aspects of PFAS removal by NF and RO membranes including evaluating different membrane products, the impact of operating conditions and water quality, and the influence of physicochemical characteristics of PFAS[10][12][13][14][15][16][17]. Most studies have focused on anionic (at neutral pH) perfluoroalkyl acid (PFAA) rejection and reported greater than 90% separation of PFAAs by available NF and RO membranes due to electrostatic and steric exclusion from the membrane polymer[10][11][13]. Water quality constituents such as organic matter and cations including calcium and magnesium have been shown to reduce rejection of PFAS[13]. However, little is known about how fouling and membrane aging impact rejection of PFAS by NF and RO membranes and additional data are needed. A recent Department of Defense ESTCP pilot scale project (ER20-5369) conducted at Colorado School of Mines (Mines) systematically evaluated the rejection of nine PFAAs by four available NF and RO products using full scale spiral-wound membrane elements in a high recovery membrane system which achieved up to 97% recovery[12]. Tight NF and the two RO membranes evaluated exhibited greater than 98% rejection of all PFAAs evaluated even at high recovery conditions (Figure 3). The loose NF membrane product evaluated provided lower than expected (based on literature) rejection of investigated PFAAs particularly at higher recovery values. These findings indicate that tight NF and RO membranes can be effective at separating PFAAs from contaminated source waters regardless of PFAA chain length. Energy requirements modeled from these experiments varied from 0.14 kWh/m3 for loose NF to 0.57 kWh/m3 for seawater RO[12].
Mines researchers have developed a mobile high-recovery closed-circuit membrane filtration system (Figure 4) that has been successfully deployed for treating groundwater at a fire training area of Wright-Patterson Air Force Base (ESTCP ER21-5136), groundwater at Peterson Space Force Base (AFCEC BAA-031), and firetruck rinsate at Tyndall Air Force Base (ESTCP ER20-5369) during recent ESTCP and AFCEC funded research projects. In these projects, NF or RO was implemented to produce a permeate stream containing low concentrations of PFAS and to concentrate PFAS into smaller volumes of retentate for subsequent destructive PFAS treatment. While NF and RO membranes have demonstrated effective rejection of PFAS, PFAS are subsequently concentrated in the membrane concentrate, or retentate stream. This concentrate stream is increasingly paired with PFAS destruction technologies, as PFAS destruction is often considered viable only for concentrated solutions of PFAS. Ongoing ESTCP funded projects include using high-recovery NF and RO to treat and concentrate groundwater leading to PFAS destruction using plasma based treatment[18] or hydrothermal alkaline treatment (HALT)[19].
Advantages and Limitations of the Technology for PFAS Removal
Advantages:
- Robust, high throughput treatment
- Mature technology with well documented solute separation performance
- High rejection of PFAS and other contaminants
- Removes solutes at the molecular scale
Limitations:
- Complex and often expensive pretreatment requirements for certain waters
- Energy intensive
- High capital costs
- Membrane fouling requiring high chemical usage for cleaning
- Concentrated waste stream requiring disposal or destruction
- Permeate quality depends on feed water concentration
- Greater operation complexity than most water treatment processes
- Water loss due to membrane separation
Summary
High-pressure membranes including NF and RO are well established technologies used in a variety of water treatment fields for the purification of water resources and industrial process waste streams. Research conducted over the past decade has demonstrated that various available membrane products can achieve high rejection of PFAS, enabling compliance with state and federal PFAS regulations. As opposed to adsorbent based PFAS removal technologies (e.g., activated carbon, ion exchange), high-pressure membranes do not have a finite capacity for PFAS removal and do not exhibit breakthrough. High-recovery membrane systems are being implemented into ex situ treatment trains to simultaneously treat PFAS impacted water resources and concentrate PFAS into the retentate stream to enable more effective and efficient PFAS destruction.
References
- ^ Zodrow, J., Arblaster, J., Conder, J., 2021. “State of the Science for Risk Assessment of PFAS at Contaminated Sites.” In: Forever Chemicals: Environmental, Economic, and Social Equity Concerns with PFAS in the Environment, edited by Kempisty, D., Racz, L., pp. 161-186. CRC Press. doi: 10.1201/9781003024521
- ^ 2.0 2.1 2.2 Cite error: Invalid
<ref>tag; no text was provided for refs namedWilf2019 - ^ 3.0 3.1 3.2 Cite error: Invalid
<ref>tag; no text was provided for refs namedBellonaEtAl2004 - ^ Cite error: Invalid
<ref>tag; no text was provided for refs namedBazarganSalgado2018 - ^ Cite error: Invalid
<ref>tag; no text was provided for refs namedTurekEtAl2017 - ^ Cite error: Invalid
<ref>tag; no text was provided for refs namedPanagopoulosEtAl2019 - ^ Cite error: Invalid
<ref>tag; no text was provided for refs namedWarsingerEtAl2018 - ^ Cite error: Invalid
<ref>tag; no text was provided for refs namedYan2017 - ^ Cite error: Invalid
<ref>tag; no text was provided for refs namedBellona2019 - ^ 10.0 10.1 10.2 Cite error: Invalid
<ref>tag; no text was provided for refs namedApplemanEtAl2013 - ^ 11.0 11.1 Cite error: Invalid
<ref>tag; no text was provided for refs namedSteinle-DarlingReinhard2008 - ^ 12.0 12.1 12.2 Cite error: Invalid
<ref>tag; no text was provided for refs namedSafulkoEtAl2023 - ^ 13.0 13.1 13.2 Liu, C.J., Strathmann, T.J., Bellona, C., 2021. Rejection of per- and polyfluoroalkyl substances (PFASs) in aqueous film-forming foam by high-pressure membranes. Water Research, 188, Article 116546. doi: 10.1016/j.watres.2020.116546
- ^ Wang, J., Wang, L., Xu, C., Zhi, R., Miao, R., Liang, T., Yue, X., Lv, Y., Liu, T., 2018. Perfluorooctane sulfonate and perfluorobutane sulfonate removal from water by nanofiltration membrane: The roles of solute concentration, ionic strength, and macromolecular organic foulants. Chemical Engineering Journal, 332, p. 787-797. doi: 10.1016/j.cej.2017.09.061
- ^ Zhao, C., Tang, C.Y., Li, P., Adrian, P., Hu, G., 2016. Perfluorooctane sulfonate removal by nanofiltration membrane—the effect and interaction of magnesium ion / humic acid. Journal of Membrane Science, 503, p. 31-41. doi: 10.1016/j.memsci.2015.12.049
- ^ Zhao, C., Zhang, J., He, G., Wang, T., Hou, D., Luan, Z., 2013. Perfluorooctane sulfonate removal by nanofiltration membrane the role of calcium ions. Chemical Engineering Journal, 233, p. 224-232. doi: 10.1016/j.cej.2013.08.027
- ^ Steinle-Darling, E., Litwiller, E., Reinhard, M., 2010. Effects of Sorption on the Rejection of Trace Organic Contaminants During Nanofiltration. Environmental Science and Technology, 44(7), p. 2592-2598. doi: 10.1021/es902846m
- ^ Richardson, S., 2021. Nanofiltration Followed by Electrical Discharge Plasma for Destruction of PFAS and Co-occurring Chemicals in Groundwater: A Treatment Train Approach. Environmental Security Technology Certification Program (ESTCP), Project ER21-5136
- ^ Bellona, C., 2023. Cradle to Grave PFAS Treatment Using Membrane and Foam Fractionation Concentration Followed by Hydrothermal Alkaline Treatment. Environmental Security Technology Certification Program (ESTCP), Project ER23-8367