Difference between revisions of "User:Jhurley/sandbox"
(→Pyrogenic Carbonaceous Matter (PCM) Enhanced Alkaline Hydrolysis) |
|||
| Line 25: | Line 25: | ||
Results of this study suggest that hydrolysis of 2,4,6-trinitrotoluene (TNT), 2,4-dinitroanisole (DNAN), and [[Wikipedia: Nitroguanidine | nitroguanidine (NQ)]], can be enhanced by the presence of various PCMs. The transformation of TNT by graphite powder, a model PCM, exhibited first-order decay kinetics, with an observed rate constant (''k<sub>obs</sub>'') of 0.258 ± 0.010 day<sup>-1</sup> and a calculated half-life (''t<sub>1/2</sub>'') of 2.70 ± 0.10 days<ref name="Ding">Ding, K., Byrnes, C., Bridge, J., Grannas, A., Xu, W., 2018. Surface-promoted hydrolysis of 2,4,6-trinitrotoluene and 2,4-dinitroanisole on pyrogenic carbonaceous matter. Chemosphere, 197, pp. 603-610. [https://doi.org/10.1016/j.chemosphere.2018.01.038 doi: 10.1016/j.chemosphere.2018.01.038]</ref>. Slower degradation was observed for DNAN under the same conditions. Increasing the pH and the temperature enhanced the degradation kinetics of DNAN<ref name="SeenthiaEtAl2024"/>. More importantly, results suggest that PCM accelerated DNAN decay by lowering the activation energy of DNAN hydrolysis by 54.3 ± 3.9%. NQ is a monoprotic acid in water with a reported ''pK<sub>a</sub>'' value of 12.8. NQ undergoes significant base hydrolysis at pH values as low as 11.5. This study showed that PCM pre-equilibrated with NQ initially accelerated NQ hydrolysis at three pH conditions (pH 11.0, 11.5, and 12.5) compared to the aqueous reaction. However, after a few hours, hydrolysis in the presence of PCM slowed down, whereas aqueous hydrolysis continued apace, indicating that the physical and chemical properties of PCMs play critical roles in controlling the hydrolysis of MCs. | Results of this study suggest that hydrolysis of 2,4,6-trinitrotoluene (TNT), 2,4-dinitroanisole (DNAN), and [[Wikipedia: Nitroguanidine | nitroguanidine (NQ)]], can be enhanced by the presence of various PCMs. The transformation of TNT by graphite powder, a model PCM, exhibited first-order decay kinetics, with an observed rate constant (''k<sub>obs</sub>'') of 0.258 ± 0.010 day<sup>-1</sup> and a calculated half-life (''t<sub>1/2</sub>'') of 2.70 ± 0.10 days<ref name="Ding">Ding, K., Byrnes, C., Bridge, J., Grannas, A., Xu, W., 2018. Surface-promoted hydrolysis of 2,4,6-trinitrotoluene and 2,4-dinitroanisole on pyrogenic carbonaceous matter. Chemosphere, 197, pp. 603-610. [https://doi.org/10.1016/j.chemosphere.2018.01.038 doi: 10.1016/j.chemosphere.2018.01.038]</ref>. Slower degradation was observed for DNAN under the same conditions. Increasing the pH and the temperature enhanced the degradation kinetics of DNAN<ref name="SeenthiaEtAl2024"/>. More importantly, results suggest that PCM accelerated DNAN decay by lowering the activation energy of DNAN hydrolysis by 54.3 ± 3.9%. NQ is a monoprotic acid in water with a reported ''pK<sub>a</sub>'' value of 12.8. NQ undergoes significant base hydrolysis at pH values as low as 11.5. This study showed that PCM pre-equilibrated with NQ initially accelerated NQ hydrolysis at three pH conditions (pH 11.0, 11.5, and 12.5) compared to the aqueous reaction. However, after a few hours, hydrolysis in the presence of PCM slowed down, whereas aqueous hydrolysis continued apace, indicating that the physical and chemical properties of PCMs play critical roles in controlling the hydrolysis of MCs. | ||
| − | == | + | ==Key Properties of PCM for MC Hydrolysis== |
| − | [[File: | + | [[File:XuFig2.png | thumb |400px| Figure 2: Proposed role of PCM properties in accelerating TNT hydrolysis<ref name="Li"/>]] |
| − | + | To investigate the effect of PCM functional group identity and pore characteristics on MC hydrolysis, a PCM-like polymer (PLP) platform was developed to allow for delineating individual PCM properties’ contributions using a reductionist approach. PLPs exhibit properties that are similar to PCM: (i) large surface area and high microporosity, (ii) highly conjugated and amorphous, and (iii) superior affinity for apolar organic contaminants. Unlike PCM, the attributes of PLP can be individually tuned and made homogeneously throughout the polymer networks. Specifically, six PLPs were synthesized via cross-coupling chemistry with specific functionality (i.e., -OH, -NH<sub>2</sub>, -N(CH<sub>3</sub>)<sub>2</sub>, and -N(CH<sub>3</sub>)<sub>3</sub><sup>+</sup>) and pore characteristics (i.e., mesopore, micropore). Different surface functional groups were incorporated into the PLPs by adapting the synthesis approach described in previous work<ref name="Li">Li, Z., Jorn, R., Samonte, P.R.V., Mao, J., Sivey, J.D., Pignatello, J.J., Xu, W., 2022. Surface-catalyzed hydrolysis by pyrogenic carbonaceous matter and model polymers: An experimental and computational study on functional group and pore characteristics. Applied Catalysis B: Environment and Energy, 319, article 121877. [https://doi.org/10.1016/j.apcatb.2022.121877 doi: 10.1016/j.apcatb.2022.121877] [[Media: LiEtAl2022.pdf | Open Access Manuscript]]</ref>. The pore characteristics of PLP were controlled by rigid node-strut topology, where two polymers were obtained, one with exclusively mesopores and the other with a mixture of micropores and mesopores. This showed that -OH and -NH<sub>2</sub> functional groups can serve as weak bases to facilitate TNT hydrolysis, whereas -N(CH<sub>3</sub>)<sub>3</sub><sup>+</sup> groups can increase the local pH by accumulating OH<sup>-</sup> near PCM surfaces. Moreover, the micropores of PCM seem capable of altering the chemical environment around TNT molecules (including their location and orientation) in such a way as to facilitate their hydrolysis. This study attempted to scrutinize the complex properties of PCM that promote surface hydrolysis, namely the functional group identity and pore characteristics. | |
| − | |||
| − | |||
==Practical Applications== | ==Practical Applications== | ||
Revision as of 12:04, 22 May 2025
Pyrogenic Carbonaceous Matter (PCM) Enhanced Alkaline Hydrolysis
High concentrations of munitions constituents (MC) residues, including legacy and insensitive high explosives (IHE), are commonly found in soil at Department of Defense (DoD) testing and training ranges, posing a significant risk to personnel and the environment. Many IHE are highly water soluble and can easily migrate from soil to groundwater and surface waters at DoD ranges. Therefore, there is a pressing need to maximize the sorption of legacy explosives and IHE, minimize their transport from DoD sites, and promote their decay whenever possible. The following article reports on a combined experimental and computational strategy to design and optimize pyrogenic carbonaceous matter (PCM, e.g., biochar and activated carbon) in ways that facilitate the retention and/or hydrolysis of legacy explosives and IHE that are of concern at DoD sites. (enviro-wiki.mtr.tsa.mybluehost.me, www.enviro-wiki.mtr.tsa.mybluehost.me)
Related Article(s):
Contributors: Dr. Wenqing Xu
Key Resource(s):
- Experimental and Computational Study of Pyrogenic Carbonaceous Matter Facilitated Hydrolysis of 2, 4-Dinitroanisole (DNAN)[1]
Introduction
Historically, pyrogenic carbonaceous matter (PCM) has been used to remove contaminants from the aqueous phase by adsorption and/or complexation, but it was not believed to facilitate their degradation. A recent Strategic Environmental Research and Development Program (SERDP) project (ER19-1239) developed evidence that PCM not only adsorbs but also catalyzes the hydrolysis of some munition constituents, thus potentially reducing the need for regeneration or replacement of the adsorbent material and thereby reducing costs associated with management of these contaminants. This project found that PCM can facilitate MC degradation on carbon surfaces and thus free up adsorption sites, allowing PCM to remove a significantly greater mass of some MCs than would be possible by adsorption alone. Any MCs such as 3-Nitro-1,2,4-triazol-5-one (NTO) that are not susceptible to alkaline hydrolysis can be safely sequestered within the carbon amendment, decreasing their bioavailability to the surrounding environment. Furthermore, the tested technology boosts alkaline hydrolysis at near-neutral pH conditions rather than high pH conditions required by current methods (i.e., lime treatment). Bench studies using soils collected from Department of Defense ranges demonstrated enhanced adsorption affinity (over three orders of magnitude) for highly mobile IHEs such as NTO because the PCM amendments maintained their reactivity over consecutive additions of IHE formulations. The findings suggest that PCM has the potential to be developed and deployed as a reactive amendment for environmental remediation of MCs. Future efforts are needed to demonstrate these materials at full scale in the field.
Using a combined experimental and computational modeling approach, the structural features of MCs and PCM that are critical for PCM-facilitated hydrolysis were identified. Employing a polymer synthesis approach, the contribution of various functional groups and pore structures in promoting MC hydrolysis were delineated. The findings of this investigation have broad implications for reactive adsorbent design and remediation. For instance, the formation of σ complexes between -NH2 surface functional groups and nitroaromatics suggests that PCM rich in -NH2 functional groups could be easily poisoned due to the irreversible binding of TNT or DNAN. By contrast, quaternary ammonium (QA) functional groups could accumulate TNT, DNAN and hydroxide (OH-) in the same spatial region, potentially enabling hydrolysis of the MCs. Therefore, efforts can be focused on populating specific functional groups on carbon amendments for groundwater and soil remediation. For example, increasing the abundance of QA groups while decreasing the presence of -NH2 and -OH can reduce the need for PCM regeneration. This is because contaminants will be destroyed on PCM surfaces rather than filling up adsorption sites. Besides functional groups, the pore structures of adsorbents could also be adjusted to favor hydrolysis and specific pathways (see Figure 3).
A novel adsorption mechanism known as charge-assisted hydrogen bond (CAHB) formation was proposed to account for the exceptionally high affinity of PCM for for MCs that do not undergo hydrolysis, such as NTO. The findings contradicted the conventional wisdom that polar organic anions (e.g., NTO) have little affinity for or are even repelled by hydrophobic carbonaceous sorbents. The results call attention to the need for new models or modification of existing models for the sorption of ionizable compounds in order to consider CAHB formation with sorbents. The findings also have potentially important implications for the use of carbons in environmental remediation more generally, particularly for strategies that enhance the retention of anionic contaminants that are otherwise highly mobile, such as nitrite, nitrate, phosphate, or some per- and polyfluoroalkyl substances (PFAS).
Feasibility of PCM-facilitated Hydrolysis of MCs
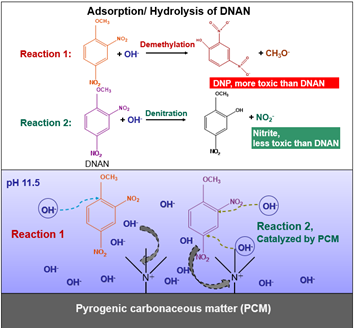
Results of this study suggest that hydrolysis of 2,4,6-trinitrotoluene (TNT), 2,4-dinitroanisole (DNAN), and nitroguanidine (NQ), can be enhanced by the presence of various PCMs. The transformation of TNT by graphite powder, a model PCM, exhibited first-order decay kinetics, with an observed rate constant (kobs) of 0.258 ± 0.010 day-1 and a calculated half-life (t1/2) of 2.70 ± 0.10 days[2]. Slower degradation was observed for DNAN under the same conditions. Increasing the pH and the temperature enhanced the degradation kinetics of DNAN[1]. More importantly, results suggest that PCM accelerated DNAN decay by lowering the activation energy of DNAN hydrolysis by 54.3 ± 3.9%. NQ is a monoprotic acid in water with a reported pKa value of 12.8. NQ undergoes significant base hydrolysis at pH values as low as 11.5. This study showed that PCM pre-equilibrated with NQ initially accelerated NQ hydrolysis at three pH conditions (pH 11.0, 11.5, and 12.5) compared to the aqueous reaction. However, after a few hours, hydrolysis in the presence of PCM slowed down, whereas aqueous hydrolysis continued apace, indicating that the physical and chemical properties of PCMs play critical roles in controlling the hydrolysis of MCs.
Key Properties of PCM for MC Hydrolysis
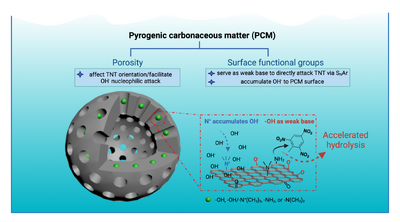
To investigate the effect of PCM functional group identity and pore characteristics on MC hydrolysis, a PCM-like polymer (PLP) platform was developed to allow for delineating individual PCM properties’ contributions using a reductionist approach. PLPs exhibit properties that are similar to PCM: (i) large surface area and high microporosity, (ii) highly conjugated and amorphous, and (iii) superior affinity for apolar organic contaminants. Unlike PCM, the attributes of PLP can be individually tuned and made homogeneously throughout the polymer networks. Specifically, six PLPs were synthesized via cross-coupling chemistry with specific functionality (i.e., -OH, -NH2, -N(CH3)2, and -N(CH3)3+) and pore characteristics (i.e., mesopore, micropore). Different surface functional groups were incorporated into the PLPs by adapting the synthesis approach described in previous work[3]. The pore characteristics of PLP were controlled by rigid node-strut topology, where two polymers were obtained, one with exclusively mesopores and the other with a mixture of micropores and mesopores. This showed that -OH and -NH2 functional groups can serve as weak bases to facilitate TNT hydrolysis, whereas -N(CH3)3+ groups can increase the local pH by accumulating OH- near PCM surfaces. Moreover, the micropores of PCM seem capable of altering the chemical environment around TNT molecules (including their location and orientation) in such a way as to facilitate their hydrolysis. This study attempted to scrutinize the complex properties of PCM that promote surface hydrolysis, namely the functional group identity and pore characteristics.
Practical Applications
The ideal use case for HALT is treating PFAS-rich liquid matrices. PFAS concentrations are high enough for HALT to be directly applicable primarily in the cases of AFFF treatment or industrial process water treatment. In the majority of use cases, it is more practical to apply a separation and concentration technology prior to HALT, to reduce the volume of liquid requiring HALT treatment while increasing PFAS concentrations in that liquid. These concentration technologies may include regenerable sorbents, membranes, or foam fractionation, all of which produce a liquid byproduct amenable for HALT.
Destruction of PFAS in Ion Exchange Regeneration Brine
One of the most promising applications of HALT is for treating PFAS-rich ion exchange (IX) regeneration brines, either in site remediation applications (e.g., groundwater treatment[4]) or industrial wastewater treatment applications[5]. IX capture and regeneration involve sorbing PFAS to an IX resin, followed by chemical desorption of PFAS from the resin, typically with a solvent and/or salt wash solution. The IX regeneration technology is commercially mature and available from several vendors.
A treatment train of IX followed by HALT shows promise for several reasons. One reason is that the HALT process is highly compatible with the liquid matrix produced through the IX regeneration. Typically, IX regeneration brine (a.k.a. “still bottoms”) contains high levels of dissolved solids such as sodium chloride, which can cause practical processing challenges with other liquid treatment technologies. However, high levels of TDS do not appear to cause processing challenges with HALT[5]. Another reason is that IX regeneration brines often contain ultra short- and short-chain PFAS, which are amenable to destructive treatment with HALT.
In 2022, commercial HALT provider Aquagga performed a bench study in partnership with the 3M Company, demonstrating PFAS destruction performance for HALT processing of a synthetic IX regeneration brine[5]. Seven treatment conditions were tested, and fluorine mass balance closure was demonstrated for most conditions using a range of analytical techniques. In 2024, Aquagga performed an on-site demonstration in partnership with the 3M Company treating IX regeneration brine produced from active wastewater treatment activities[6].
Foam Fractionate Treatment
Foam fractionation is a technology that concentrates PFAS in liquids by taking advantage of the hydrophobic/interface-partitioning behavior exhibited by many types of PFAS. Foam fractionation is seeing broad adoption for challenging liquid matrices such as landfill leachate and groundwater. Long-chain PFAS are known to partition to interfaces much more readily than short-chain PFAS, and foam fractionation is correspondingly much more effective at removing long-chain PFAS from liquids. When coupled with HALT, foam fractionation can remove and destroy a high fraction of PFAS from challenging liquid matrices[7].
Destruction of PFAS in AFFF
Legacy AFFF contains high levels of PFAS (typically 0.1 to 6 wt%) in a liquid matrix. Several studies at lab and pilot scales have demonstrated that HALT can destroy PFAS in AFFF with minimal dilution[8]. While the treatment is effective, the wide variety of AFFF formulations make this a challenging application.
Advantages and Drawbacks
Advantages of HALT include:
- Ability to achieve >99% destruction of all PFAS chain lengths and subtypes
- Ability to fully mineralize or defluorinate PFAS to dissolved inorganic fluoride as an end product
- Commercial systems are compact and simple to operate
- Commercial systems do not have an air emission point
- Ability to treat wastes with high TDS
- Ability to treat wastes with high TOC
- Low overall energy usage (<0.9 kWh/gal-treated)
Drawbacks or challenges associated with HALT include:
- Not well-suited for directly processing solid materials or slurries
- Treated effluent brine contains high TDS and must be managed accordingly
- Hard minerals (e.g., Ca2+) may precipitate and require periodic cleaning
- The use of strong bases and conjugate acids require safe chemical handling practices external to the HALT system and appropriate operator precautions
- High-pressure, high-temperature, and high-pH operating conditions are harsh and corrosive on processing equipment, and appropriate material selection, metallurgy, and corrosion control methods must be applied to ensure reactor vessel reliability
References
- ^ 1.0 1.1 1.2 Seenthia, N.I., Bylaska, E.J., Pignatello, J.J., Tratnyek, P.G., Beal, S.A., Xu, W., 2024. Experimental and Computational Study of Pyrogenic Carbonaceous Matter Facilitated Hydrolysis of 2, 4-Dinitroanisole (DNAN). Environmental Science and Technology, 58(21), pp. 9404–9415. doi: 10.1021/acs.est.4c01069 Open Access pdf
- ^ Ding, K., Byrnes, C., Bridge, J., Grannas, A., Xu, W., 2018. Surface-promoted hydrolysis of 2,4,6-trinitrotoluene and 2,4-dinitroanisole on pyrogenic carbonaceous matter. Chemosphere, 197, pp. 603-610. doi: 10.1016/j.chemosphere.2018.01.038
- ^ 3.0 3.1 Li, Z., Jorn, R., Samonte, P.R.V., Mao, J., Sivey, J.D., Pignatello, J.J., Xu, W., 2022. Surface-catalyzed hydrolysis by pyrogenic carbonaceous matter and model polymers: An experimental and computational study on functional group and pore characteristics. Applied Catalysis B: Environment and Energy, 319, article 121877. doi: 10.1016/j.apcatb.2022.121877 Open Access Manuscript
- ^ Pinkard, B.R., 2024. Hydrothermal Alkaline Treatment for a Closed-Loop, On-Site PFAS Treatment Solution. Project Number ER23-8400, Environmental Security Technology Certification Program (ESTCP). Project Website
- ^ 5.0 5.1 5.2 Cite error: Invalid
<ref>tag; no text was provided for refs namedPinkardEtAl2024a - ^ Cite error: Invalid
<ref>tag; no text was provided for refs namedPinkardEtAl2024b - ^ Cite error: Invalid
<ref>tag; no text was provided for refs namedHaoEtAl2023 - ^ Cite error: Invalid
<ref>tag; no text was provided for refs namedHaoEtAl2021
