Difference between revisions of "Sediment Capping"
| Line 7: | Line 7: | ||
*Sediment Risk Assessment | *Sediment Risk Assessment | ||
*[[Passive Sampling of Sediments]] | *[[Passive Sampling of Sediments]] | ||
| + | |||
'''Contributor(s):''' Dr. Danny Reible | '''Contributor(s):''' Dr. Danny Reible | ||
| + | |||
'''Key Resource(s):''' | '''Key Resource(s):''' | ||
Revision as of 22:38, 29 November 2021
Capping is an in situ remedial technology that involves placement of a clean substrate on the surface of contaminated sediments to reduce contaminant uptake by benthic organisms and contaminant flux to surface water. Simple sand caps can be effective in reducing exposure of benthic organisms and in limiting oxygen transport into the contaminated sediments, resulting in precipitation of metal sulfides. Amendments are sometimes included in caps to reduce cap permeability and groundwater upwelling, to increase contaminant sorption or biodegradation, or to improve habitat.
Related Article(s):
- Contaminated Sediments - Introduction
- In Situ Treatment of Contaminated Sediments with Activated Carbon
- Sediment Risk Assessment
- Passive Sampling of Sediments
Contributor(s): Dr. Danny Reible
Key Resource(s):
- Processes, Assessment and Remediation of Contaminated Sediments[1]
- Guidance for In-Situ Subaqueous Capping of Contaminated Sediments[2]
Introduction
Capping is an in situ remedial technology for contaminated sediments that involves placement of a clean substrate on the sediment surface. Capping contaminated sediments following dredging operations and capping of dredged material to stabilize contaminants has been a common practice by the United States Army Corps of Engineers since the 1970s. Beginning in the 1980s, in Japan and subsequently elsewhere, capping has been used more widely as a remedial approach to improve the quality of the bottom substrate and reduce contaminant exposures to benthic organisms and fish. The USEPA published a capping guidance document in 1998 that summarizes past uses of sediment capping and outlines its basic design[2]. Although capping technology has developed substantially in the past 20 years, this early reference still provides useful information on the approach and its applications. A more recent summary of capping is described in Reible 2014[1].
Capping serves to contain contaminated sediment solids, isolate contaminants from benthic organisms and reduce contaminant transport to the sediment surface and overlying water. The clean substrate may be an inert material such as sand, a natural sorbing material such as other sediments or clays, or be amended with an active/reactive material to enhance the isolation of the contaminants. Amendments to enhance contaminant isolation include permeability reduction agents to divert groundwater flow, sorbents to retard contaminant migration through the capping layer or provide greater accumulation capacity, or reagents to encourage degradation or transformation of the contaminants.
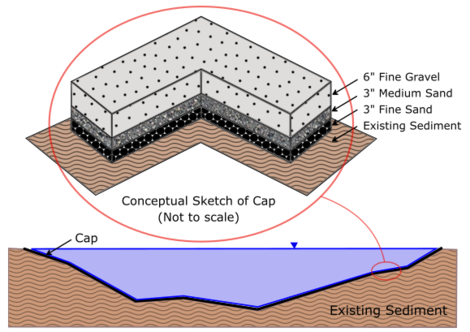
The basic concept of a cap is illustrated in Figure 1. The Figure also illustrates that a cap is often a thin layer or layers relative to water depth and generally causes little disturbance to the underlying sediments or body of water in which it is placed. Depending upon the erosive forces to which the cap may be subjected, the surface layer may be composed of relatively coarse material to withstand those erosive forces.
Although a cap is typically thin compared to the water depth, it generally must be thicker than the biologically active zone (BAZ) of the sediments. The biologically active zone is that zone in which benthic organisms live and interact with the sediment. Their activities tend to mix the BAZ (known as bioturbation) over the course of a few years and thus a cap that is thinner than the BAZ will tend to become intermixed with the underlying contaminated sediments. Processes other than bioturbation including diffusion, advection or groundwater upwelling, hyporheic exchange near the interface, biogenic gas production and migration and underlying sediment consolidation can all lead to contaminant migration into and through a cap. These occur at different rates and intensities and their assessment and evaluation ultimately governs the effectiveness of a cap and the feasibility of its use as a sediment remediation technology for a particular site.
In general, capping is an effective remedial technology for contaminants that are strongly associated with the sediment solids including hydrophobic organic compounds such as high molecular weight polycyclic aromatic hydrocarbons (PAHs), polychlorinated biphenyls (PCBs), dioxins and DDTx, but also heavy metals. Hydrophobic organic compounds tend to strongly associate with the organic fraction of sediments so organic rich sediments or the addition of organic phases to the capping material can be very effective at containing these contaminants. Many of the common heavy metals of concern, including cadmium, copper, nickel, zinc, lead and mercury, tend to be associated with insoluble sulfides under strongly reducing conditions. Since oxygen penetration into a capping layer is typically limited to a few cm or less at the surface, a cap serves to drive the underlying contaminated sediment toward strongly reducing conditions and, particularly in marine and estuarine sediments, encourage sulfate reduction leading to the formation of these insoluble sulfides. The low solubility of these sulfides encourages retention by a capping layer and makes the cap extremely effective as a remedial approach for sediments with elevated concentrations of heavy metals.
A variety of tools have been developed to evaluate the processes leading to sorption and retardation of contaminants as well as processes leading to contaminant migration and release. The original references quantifying contaminant behavior in a sediment cap were explored in a series of papers in the early 1990s[3][4]. Since that time, design tools have been continuously improved. CapSim is a commonly used and current tool developed by Dr. Reible and collaborators. This tool can evaluate contaminant release from uncapped, capped, and treated sediments for purposes of design and evaluation. The model formulation and structure is described in Shen et al. 2018[5]. One common use of such a tool is to evaluate the effect of various cap materials and thicknesses on the performance of a cap.
Cap Design and Materials for Chemical Containment
An inert material such as sand can be effective as a capping material where contaminants are strongly associated with solids and where the operative site specific transport mechanisms do not lead to rapid contaminant migration through such a material. Additional contaminant containment can often be achieved through the placement of clean sediment, e.g. dredged material from a nearby location. Other materials as cap layers or amendments may be useful to address particularly mobile contaminants or when particular degradative mechanisms can be exploited. The Anacostia River was the site of a demonstration that first tested “active” or “amended” capping in the field[6][7]. Amended caps are often the best option when groundwater upwelling or other advective processes promote significant mobility of contaminants and the addition of sorbents can slow that contaminant migration[8]. Although a variety of materials have been proposed for sediment caps, a far smaller number of options have been successfully employed in the field.
Metals migration is very site dependent due to the potential for many metals to complex with other species in the interstitial water and the specific metal speciation present at a site. Often, the strongly reducing environment beneath a cap renders many common metals unavailable through the formation of metal sulfides. In such cases, a simple sand cap can be very effective. Amended caps to manage metal contaminated sediments may be advantageous when site specific conditions lead to elevated metals mobility, but should be supported with site specific testing[9].
For hydrophobic organic contaminants, cap amendments that directly control groundwater upwelling and also sorbents that can remove migrating contaminants from that groundwater have been successfully employed. Examples include clay materials such as AquaBlok® for permeability control, sorbents such as activated carbon for truly dissolved contaminants, and organophilic clays for separate phase contaminants.
The placement of clean sediment as an in situ cap can be difficult when the material is fine grained or has a low density. Capping with a layer of coarse grained material such as clean sand mitigates this issue although clean sands have minimal sorption capacity. Because of this limitation, sand caps may not be sufficient for achieving remedial goals in sites where contamination levels are high or transport rates are fast due to pore water upwelling or tidal pumping effects. Conditions such as these may require the use of “active” amendments to reduce transport rates.
Capping with clean sand provides a physical barrier between the underlying contaminated material and the overlying water, stabilizes the underlying sediment to prevent re-suspension of contaminated particles, and can reduce chemical exposure under certain conditions. Sand primarily provides a passive barrier to the downward penetration of bioturbating organisms and the upward movement of sediment or contaminants. Although conventional sandy caps can often be an effective means of managing contaminated sediments, there are conditions when sand caps may not be capable of achieving design objectives. Some factors that reduce the effectiveness of sand caps include:
- erosion and loss of cap integrity
- high groundwater upwelling rates
- mobile (low sorption) contaminants of concern (COCs)
- high COC concentrations
- unusually toxic COCs
- the presence of tidal influences
- the presence of non-aqueous phase liquids (NAPLs)
- high rates of gas ebullition
Of these, the first three are common limitations to capping and often control the ability to effectively design and implement a cap as a sediment remedial strategy. In these cases, it may be possible to offset these issues by increasing the thickness of the cap. However, the required thickness can reach infeasible levels in shallow streams or navigable water bodies. In addition, increased construction costs associated with thick caps may become prohibitive. As a result of these issues, caps that use alternative materials (also known as active caps) to reduce the thickness or increase the protectiveness of a cap may be necessary. The materials in active caps are designed to interact with the COCs to enhance the containment properties of the cap.
Apatites are a class of naturally occurring minerals that have been investigated as a sorbent for metals in soils and sediments[10][6][11]. Apatites consist of a matrix of calcium phosphate and various other common anions, including fluoride, chloride, hydroxide, and occasionally carbonate. Metals are sequestered either through direct ion exchange with the calcium atom or dissolution of hydroxyapatite followed by precipitation of lead apatite. Zeolites, which are microporous aluminosilicate minerals with a high cationic exchange capacity (CEC), have also been proposed to manage metal species[12].
It is possible to create a hydrophobic, sorbing layer for non-polar organics by exchanging a cationic surfactant onto the surface of clays such as zeolites and bentonites,. Organoclay is a modified bentonite containing such substitutions that has been evaluated for control of non-aqueous phase NAPLs and other organic contaminants[13]. An organoclay cap has been implemented for sediment remediation at the McCormick and Baxter site in Portland, OR[14]. A similar organic sorbing phase can be formed by treating zeolites with surfactants but this approach has not been reported for contaminated sediments.
Activated carbon is a strong sorbent of hydrophobic organic compounds and has been used as a treatment for sediments or as an active sorbent within a capping layer[15][16][17][18][19]. Placement of activated carbon for sediment capping is difficult due to the near neutral buoyancy of the material but it has been applied in this manner in relatively low energy environments such as Onondaga Lake, Syracuse, NY[20]. Alternatives in higher energy environments include placement of activated carbon in a mat such as the CETCO Reactive Core Mat (RCM)® or Huesker Tektoseal®, or as a composite material such as SediMite® or AquaGate®. In the case of the mats, powdered or granular activated carbon can be placed in a controlled layer while the density of the composite materials is such that they can be broadcast from the surface and allowed to settle to the bottom. In a sediment treatment application, the composite material would either be worked into the surface or allowed to intermix gradually by bioturbation and other processes. In a capping application, the mat or composite material would typically be combined or overlain with a sand or other capping layer to keep it in place and to provide a chemical isolation layer away from the sediment surface.
As an alternative to a sorptive capping amendment, low-permeability cap amendments have been proposed to enhance cap design life by decreasing pore water advection. Low permeability clays are an effective means to divert upwelling groundwater away from a contaminated sediment area but are difficult to place in the aqueous environment. Bentonite clays can be placed in mats similar to what is done to provide a low permeability liner in landfills. There are also commercial products that can place clays directly such as the composite material AquaBlok®, a bentonite clay and polymer based mineral around an aggregate core[21].
Sediment caps become colonized by microorganisms from the sediments and surface water and potentially become a zone of pollutant biotransformation over time. Aerobic degradation occurs only near the solids-water interface in which benthic organisms are active and thus there might still be significant benthic organism exposure to contaminants. Biotransformation in the anaerobic zone of a cap, which typically extends well beyond the zone of benthic activity, could significantly reduce the risk of pollutant exposure but successful caps encouraging deep degradation processes have not been demonstrated beyond the laboratory. The addition of materials such as nutrients and oxygen releasing compounds for enhancing the attenuation of contaminants through biodegradation has also been assessed but not applied in the field. Short term improvements in biodegradation rates can be achieved through tailoring of conditions or addition of nutrients but long term efficacy has not been demonstrated[22].
Cap Design and Materials for Habitat Restoration
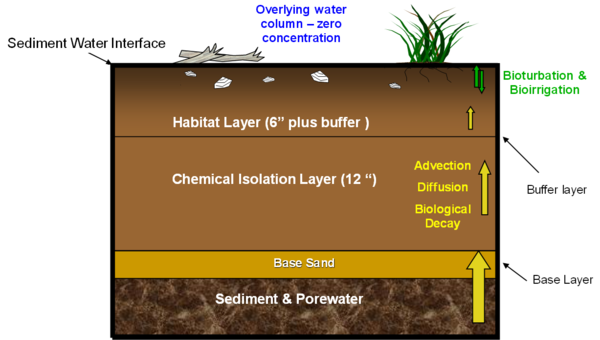
In addition to providing chemical isolation and containment, a cap can also be used to provide improvements for organisms by enhancing the habitat characteristics of the bottom substrate[23][24][20]. Often, contaminated sediment environments are degraded for a variety of reasons in addition to the toxic constituents. One way to overcome this is to provide both a habitat layer and chemical isolation or contaminant capping layer. Figure 2 illustrates just such a design providing a more appropriate habitat enhancing substrate, in this case by incorporation of additional organic material, vegetation and debris, which is often used by fish species for protection, into the surface layer. In a high energy environment, it should be recognized that it may not be possible to keep a suitable habitat layer in place during high flow events. This would be true of suitable habitat that had developed naturally as well as a constructed habitat layer and it is presumed that if such a habitat is the normal condition of the waterbody that it will recover over time between such high flow events.
Summary
Clean substrate can be placed at the sediment-water interface for the purposes of reducing exposure to and risk from contaminants in the sediments. The cap can consist of simple materials such as sand designed to physically stabilize contaminated sediments and separate the benthic community from those contaminants or may include other materials designed to sequester contaminants even under adverse conditions including strong groundwater upwelling or highly mobile contaminants. The surface of a cap may be designed of coarse material such as gravel or cobble to be stable under high flow events or designed to be more appropriate habitat for benthic and aquatic organisms. As a result of its flexibility, simplicity and low cost relative to its effectiveness, capping is one of the most prevalent remedial technologies for sediments.
References
- ^ 1.0 1.1 Reible, D. D., Editor, 2014. Processes, Assessment and Remediation of Contaminated Sediments. Springer, New York, NY. 462 pp. ISBN: 978-1-4614-6725-0
- ^ 2.0 2.1 Palermo, M., Maynord, S., Miller, J. and Reible, D., 1998. Guidance for In-Situ Subaqueous Capping of Contaminated Sediments. Assessment and Remediation of Contaminated Sediments (ARCS) Program, Great Lakes National Program Office, US EPA 905-B96-004. 147 pp. Report.pdf
- ^ Wang, X.Q., Thibodeaux, L.J., Valsaraj, K.T. and Reible, D.D., 1991. Efficiency of Capping Contaminated Bed Sediments in Situ. 1. Laboratory-Scale Experiments on Diffusion-Adsorption in the Capping Layer. Environmental Science and Technology, 25(9), pp.1578-1584. DOI: 10.1021/es00021a008
- ^ Thoma, G.J., Reible, D.D., Valsaraj, K.T. and Thibodeaux, L.J., 1993. Efficiency of Capping Contaminated Bed Sediments in Situ 2. Mathematics of Diffusion-Adsorption in the Capping Layer. Environmental Science and Technology, 27(12), pp.2412-2419. DOI: 10.1021/es00048a015
- ^ Shen, X., Lampert, D., Ogle, S. and Reible, D., 2018. A software tool for simulating contaminant transport and remedial effectiveness in sediment environments. Environmental Modelling and Software, 109, pp. 104-113. DOI: 10.1016/j.envsoft.2018.08.014
- ^ 6.0 6.1 Reible, D., Constant, D.W., Roberts, K. and Zhu, Y., 2003. Active capping demonstration project in anacostia DC. In Second International Conference on the Remediation of Contaminated Sediments: October. Free download available from: ResearchGate
- ^ Reible, D., Lampert, D., Constant, D., Mutch Jr, R.D. and Zhu, Y., 2006. Active Capping Demonstration in the Anacostia River, Washington, DC. Remediation Journal: The Journal of Environmental Cleanup Costs, Technologies and Techniques, 17(1), pp. 39-53. DOI: 10.1002/rem.20111
- ^ Ghosh, U., Luthy, R.G., Cornelissen, G., Werner, D. and Menzie, C.A., 2011. In-situ Sorbent Amendments: A New Direction in Contaminated Sediment Management. Environmental Science and Technology, 45(4), pp. 1163-1168. Article.pdf
- ^ Viana, P.Z., Yin, K. and Rockne, K.J., 2008. Modeling Active Capping Efficacy. 1. Metal and Organometal Contaminated Sediment Remediation. Environmental Science and Technology, 42(23), pp. 8922-8929. DOI: 10.1021/es800942t
- ^ Melton, J.S., Crannell, B.S., Eighmy, T.T., Wilson, C. and Reible, D.D., 2003. Field Trial of the UNH Phosphate-Based Reactive Barrier Capping System for the Anacostia River. EPA Grant R819165-01-0
- ^ Knox, A.S., Paller, M.H. and Roberts, J., 2012. Active Capping Technology—New Approaches for In Situ Remediation of Contaminated Sediments. Remediation Journal, 22(2), pp.93-117. DOI: 10.1002/rem.21313 Free download available from: ResearchGate
- ^ Zhan, Y., Yu, Y., Lin, J., Wu, X., Wang, Y. and Zhao, Y., 2019. Simultaneous control of nitrogen and phosphorus release from sediments using iron-modified zeolite as capping and amendment materials. Journal of Environmental Management, 249, p.109369. DOI: 10.1016/j.jenvman.2019.109369
- ^ Reible, D.D., Lu, X., Moretti, L., Galjour, J. and Ma, X., 2007. Organoclays for the capping of contaminated sediments. AIChE Annual Meeting. ISBN: 978-081691022-9
- ^ Parrett, K. and Blishke, H., 2005. 23-Acre Multilayer Sediment Cap in Dynamic Riverine Environment Using Organoclay an Adsorptive Capping Material. Presentation to Society of Environmental Toxicology and Chemistry (SETAC), 26th Annual Meeting.
- ^ Zimmerman, J.R., Ghosh, U., Millward, R.N., Bridges, T.S. and Luthy, R.G., 2004. Addition of Carbon Sorbents to Reduce PCB and PAH Bioavailability in Marine Sediments: Physicochemical Tests. Environmental Science and Technology, 38(20), pp. 5458-5464. DOI: 10.1021/es034992v
- ^ Werner, D., Higgins, C.P. and Luthy, R.G., 2005. The sequestration of PCBs in Lake Hartwell sediment with activated carbon. Water Research, 39(10), pp. 2105-2113. DOI: 10.1016/j.watres.2005.03.019
- ^ Abel, S. and Akkanen, J., 2018. A Combined Field and Laboratory Study on Activated Carbon-Based Thin Layer Capping in a PCB-Contaminated Boreal Lake. Environmental Science and Technology, 52(8), pp. 4702-4710. DOI: 10.1021/acs.est.7b05114 Article.pdf
- ^ Payne, R.B., Ghosh, U., May, H.D., Marshall, C.W. and Sowers, K.R., 2019. A Pilot-Scale Field Study: In Situ Treatment of PCB-Impacted Sediments with Bioamended Activated Carbon. Environmental Science and Technology, 53(5), pp. 2626-2634. DOI: 10.1021/acs.est.8b05019
- ^ Yan, S., Rakowska, M., Shen, X., Himmer, T., Irvine, C., Zajac-Fay, R., Eby, J., Janda, D., Ohannessian, S. and Reible, D.D., 2020. Bioavailability Assessment in Activated Carbon Treated Coastal Sediment with In situ and Ex situ Porewater Measurements. Water Research, 185, p. 116259. DOI: 10.1016/j.watres.2020.116259
- ^ 20.0 20.1 Vlassopoulos, D., Russell, K., Larosa, P., Brown, R., Mohan, R., Glaza, E., Drachenberg, T., Reible, D., Hague, W., McAuliffe, J. and Miller, S., 2017. Evaluation, Design, and Construction of Amended Reactive Caps to Restore Onondaga Lake, Syracuse, New York, USA. Journal of Marine Environmental Engineering, 10(1), pp. 13-27. Free download available from: ResearchGate
- ^ Barth, E.F., Reible, D. and Bullard, A., 2008. Evaluation of the physical stability, groundwater seepage control, and faunal changes associated with an AquaBlok® sediment cap. Remediation: The Journal of Environmental Cleanup Costs, Technologies and Techniques, 18(4), pp.63-70. DOI: 10.1002/rem.20183
- ^ Pagnozzi, G., Carroll, S., Reible, D.D. and Millerick, K., 2020. Biological Natural Attenuation and Contaminant Oxidation in Sediment Caps: Recent Advances and Future Opportunities. Current Pollution Reports, pp.1-14. DOI: 10.1007/s40726-020-00153-5
- ^ Yozzo, D.J., Wilber, P. and Will, R.J., 2004. Beneficial use of dredged material for habitat creation, enhancement, and restoration in New York–New Jersey Harbor. Journal of Environmental Management, 73(1), pp. 39-52. DOI: 10.1016/j.jenvman.2004.05.008
- ^ Zhang, C., Zhu, M.Y., Zeng, G.M., Yu, Z.G., Cui, F., Yang, Z.Z. and Shen, L.Q., 2016. Active capping technology: a new environmental remediation of contaminated sediment. Environmental Science and Pollution Research, 23(5), pp.4370-4386. DOI: 10.1007/s11356-016-6076-8