Difference between revisions of "User:Jhurley/sandbox"
(→Toxicological Effects of PFAS) |
(→Toxicological Effects of PFAS) |
||
| Line 43: | Line 43: | ||
Toxicity assessments are ongoing for the following PFAS: | Toxicity assessments are ongoing for the following PFAS: | ||
*Perfluorononanoic acid (PFNA) | *Perfluorononanoic acid (PFNA) | ||
| − | *Perfluorodecanoic acid (PFDA) | + | *Perfluorodecanoic acid (PFDA) |
It is important to note human health toxicity criteria for inhalation of PFAS are not included in the Final Toxicological Reviews and are not currently available. | It is important to note human health toxicity criteria for inhalation of PFAS are not included in the Final Toxicological Reviews and are not currently available. | ||
In addition to IRIS, state agencies have developed peer-reviewed provisional toxicity values that have been incorporated into USEPA’s RSLs, which are updated biannually. These values have not been reviewed by or incorporated into IRIS. | In addition to IRIS, state agencies have developed peer-reviewed provisional toxicity values that have been incorporated into USEPA’s RSLs, which are updated biannually. These values have not been reviewed by or incorporated into IRIS. | ||
| + | |||
| + | With respect to ecological toxicity, effects on reproduction, growth, and development of avian and mammalian wildlife have been documented in controlled laboratory studies of exposures of standard toxicological test species (e.g., mice, quail) to PFAS. Many of these studies have been reviewed<ref name="ConderEtAl2020"> Conder, J., Arblaster, J., Larson, E., Brown, J., Higgins, C., 2020. Guidance for Assessing the Ecological Risks of PFAS to Threatened and Endangered Species at Aqueous Film Forming Foam-Impacted Sites. Strategic Environmental Research and Development Program (SERDP) Project ER 18-1614. [https://serdp-estcp.mil/projects/details/3f890c9b-7f72-4303-8d2e-52a89613b5f6 Project Website] [[Media: ER18-1614_Guidance.pdf | Guidance Document]]</ref><ref name="GobasEtAl2020">Gobas, F.A.P.C., Kelly, B.C., Kim, J.J., 2020. Final Report: A Framework for Assessing Bioaccumulation and Exposure Risks of PFAS in Threatened and Endangered Species on AFFF-Impacted Sites. SERDP Project ER18-1502. [https://serdp-estcp.mil/projects/details/09c93894-bc73-404a-8282-51196c4be163 Project Website] [[Media: ER18-1502_Final.pdf | Final Report]]</ref><ref name="Suski2020">Suski, J.G., 2020. Investigating Potential Risk to Threatened and Endangered Species from Per- and Polyfluoroalkyl Substances (PFAS) on Department of Defense (DoD) Sites. SERDP Project ER18-1626. [https://serdp-estcp.mil/projects/details/c328f8e3-95a4-4820-a0d4-ef5835134636 Project Website] [[Media: ER18-1626_Final.pdf | Report.pdf]]</ref><ref name="ZodrowEtAl2021a">Zodrow, J.M., Frenchmeyer, M., Dally, K., Osborn, E., Anderson, P. and Divine, C., 2021. Development of Per and Polyfluoroalkyl Substances Ecological Risk-Based Screening Levels. Environmental Toxicology and Chemistry, 40(3), pp. 921-936. [https://doi.org/10.1002/etc.4975 doi: 10.1002/etc.4975] [[Media: ZodrowEtAl2021a.pdf | Open Access Article]]</ref> to derive ecological Toxicity Reference Values (TRVs). TRVs can be used alongside exposure information and other considerations to assess ecological risk. Avian and mammalian wildlife receptors are generally expected to have the highest risks due to PFAS exposure. Direct toxicity to aquatic life, such as fish and invertebrates, from exposure to sediment and surface water also occurs, though concentrations in water associated with adverse effects to aquatic life are generally higher than those that could result in adverse effects to aquatic-dependent wildlife. Soil invertebrates and plants are less sensitive to PFAS when compared to terrestrial wildlife, with risk-based PFAS concentrations in soil being much higher than those associated with potential effects to terrestrial wildlife<ref name="ZodrowEtAl2021a"/>. | ||
Revision as of 15:16, 8 October 2025
PFAS Toxicology and Risk Assessment
This article presents an overview of current practices for human health and ecological risk assessment related to per- and poly-fluoroalkyl substances (PFAS) exposures at aqueous film-forming foam (AFFF) impacted sites.
Related Article(s):
Contributors: Jennifer Arblaster, Jason Conder, Jean Zodrow and Elizabeth Nichols
Key Resource(s):
- State of the Science for Risk Assessment of PFAS at Contaminated Sites[1]
- Interstate Technology Regulatory Council (ITRC), PFAS – Per- and Polyfluoroalkyl Substances
PFAS Exposure and Conceptual Site Models

This article provides a brief overview of the environmental toxicology and risk assessment of per- and polyfluoroalkyl substances (PFAS). The article’s main focus is on the environmental toxicology and risk assessment of PFAS derived from aqueous film-forming foam (AFFF).
The use of AFFF can release PFAS into the environment during fire training, an emergency response, or as a result of leaks or spills from AFFF systems. Following AFFF releases, perfluoroalkyl acids (PFAAs), particularly PFOS, PFOA, and PFHxS, tend to be the most commonly detected PFAS in environmental media. Due to their solubility, sorption, and bioaccumulation properties, perfluoroalkyl carboxylic acids (PFCAs) and perfluoroalkyl sulfonic acids (PFSAs) can be prevalent in a variety of environmental media, including groundwater, surface water, soil, sediment, biosolids, landfill leachate, plants, fish, invertebrates, and wildlife[3].
PFAS exhibit a range of physical and chemical properties, with the fate of the PFAAs, particularly the PFCAs and PFSAs, being the most studied PFAS. PFAAs are relatively water-soluble and mobile in the environment, are not volatile (i.e., they do not evaporate to the atmosphere readily[4]) and can sorb to the organic carbon present in soil or sediment. PFAAs are more soluble and mobile compared to other persistent organic chemicals of concern documented at contaminated sites. PFAS can bioaccumulate in animals and plants, and persistent PFAS, such as PFCAs and PFSAs, do not undergo significant biodegradation or biotransformation once present in a biological system[5].
The current state of the science and understanding of PFAS fate and transport indicates that the human health issues associated with PFAS AFFF sites are primarily the exposure pathways associated with drinking water ingestion and dietary intake of PFAS[1]. Incidental soil ingestion and/or dust inhalation are typically of moderate concern and are recommended for inclusion into human health risk assessments, but compared to drinking water and dietary ingestion, generally result in lower exposures for most receptors. Exposures via dermal contact with soils and water, and inhalation of vapors (due to volatilization of PFAS), are generally of even lower concern for most sites with AFFF PFAS sources. Human health conceptual site models (CSMs) for AFFF sites typically reflect common receptors including current or future residents and industrial or commercial workers, depending on the current and reasonable anticipated future land uses at the site, along with potential exposures in offsite areas. Receptors associated with recreation and fishing activities may be incorporated if water resources used for recreational purposes are located near the site. Additional considerations may need to be incorporated into the CSM, such as the source of PFAS release into the environment. Release mechanism can differ based on site uses of PFAS. For example, while AFFF use often resulted in historic releases to ground surfaces, industrial emissions can result in aerial deposition, and biosolids application can result in widespread releases to soils which result in different exposure pathways that should be considered.
Ecological CSMs generally focus on exposures in areas adjacent to or downgradient of initial AFFF releases which have habitats present which support ecological resources (Figure 1). Most areas at the point of AFFF releases (and many industrial areas where PFAS products are or were used) do not generally feature favorable ecological habitats that make these areas relevant for ecological risk assessment. However, the relatively high solubility of PFAS in water results in a high potential for offsite transport via groundwater, surface water and stormwater, or by erosion of impacted soils and sediment[2].
Toxicological Effects of PFAS
The characterization of toxicological effects in human health risk assessments is based on toxicological studies of mammalian exposures to per- and polyfluoroalkyl substances (PFAS), primarily studies involving perfluorooctanesulfonic acid (PFOS) and perfluorooctanoic acid (PFOA). The most sensitive noncancer adverse effects involve the liver and kidney, immune system, and various developmental and reproductive endpoints[6]. A select number of PFAS have been evaluated for carcinogenicity, primarily using epidemiological data. Only PFOS and PFOA (and their derivatives) have sufficient data for USEPA to characterize as Likely to Be Carcinogenic to Humans via the oral route of exposure. Epidemiological studies provided evidence of bladder, prostate, liver, kidney, and breast cancers in humans related to PFOS exposure, as well as kidney and testicular cancer in humans and limited evidence of breast cancer related to PFOA exposure[6][7][8].
USEPA’s Integrated Risk Management System (IRIS) Program is developing Toxicological Reviews to improve understanding of the toxicity of several additional PFAS (i.e., not solely PFOA and PFOS). Toxicological Reviews provide an overview of cancer and noncancer health effects based on current literature and, where data are sufficient, derive human health toxicity criteria (i.e., human health oral reference doses and cancer slope factors) that form the basis for risk-based decision making. For risk assessors, these documents provide USEPA reference doses and cancer slope factors that can be used with exposure information and other considerations to assess human health risk. Final Toxicological Reviews have been completed for the following PFAS:
- Perfluorooctanesulfonic acid (PFOS)
- Perfluorooctanoic acid (PFOA)
- Perfluorobutanoic acid (PFBA)
- Perfluorohexanoic acid (PFHxA)
- Perfluorobutane sulfonic acid (PFBS)
- Perfluoropropionic acid (PFPrA)
- Perfluorohexane sulfonic acid (PFHxS)
- Lithium bis[(trifluoromethyl)sulfonyl]azanide (HQ-115)
- Hexafluoropropylene oxide dimer acid (HFPO DA) and its Ammonium Salt
Toxicity assessments are ongoing for the following PFAS:
- Perfluorononanoic acid (PFNA)
- Perfluorodecanoic acid (PFDA)
It is important to note human health toxicity criteria for inhalation of PFAS are not included in the Final Toxicological Reviews and are not currently available. In addition to IRIS, state agencies have developed peer-reviewed provisional toxicity values that have been incorporated into USEPA’s RSLs, which are updated biannually. These values have not been reviewed by or incorporated into IRIS.
With respect to ecological toxicity, effects on reproduction, growth, and development of avian and mammalian wildlife have been documented in controlled laboratory studies of exposures of standard toxicological test species (e.g., mice, quail) to PFAS. Many of these studies have been reviewed[9][10][11][12] to derive ecological Toxicity Reference Values (TRVs). TRVs can be used alongside exposure information and other considerations to assess ecological risk. Avian and mammalian wildlife receptors are generally expected to have the highest risks due to PFAS exposure. Direct toxicity to aquatic life, such as fish and invertebrates, from exposure to sediment and surface water also occurs, though concentrations in water associated with adverse effects to aquatic life are generally higher than those that could result in adverse effects to aquatic-dependent wildlife. Soil invertebrates and plants are less sensitive to PFAS when compared to terrestrial wildlife, with risk-based PFAS concentrations in soil being much higher than those associated with potential effects to terrestrial wildlife[12].
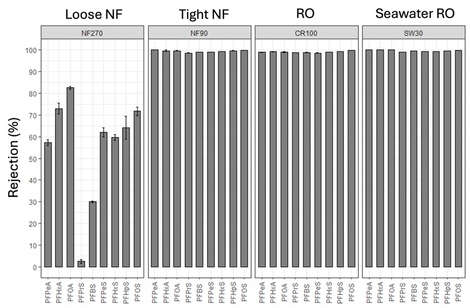
The effectiveness of RO and NF membranes for dissolved solute rejection has led to high-pressure membranes being regarded as one of the best available technologies for PFAS removal for over a decade[13][14]. Several studies have evaluated aspects of PFAS removal by NF and RO membranes including evaluating different membrane products, the impact of operating conditions and water quality, and the influence of physicochemical characteristics of PFAS[13][15][16][17][18][19][20]. Most studies have focused on anionic (at neutral pH) perfluoroalkyl acid (PFAA) rejection and reported greater than 90% separation of PFAAs by available NF and RO membranes due to electrostatic and steric exclusion from the membrane polymer[13][14][16]. Water quality constituents such as organic matter and cations including calcium and magnesium have been shown to reduce rejection of PFAS[16]. However, little is known about how fouling and membrane aging impact rejection of PFAS by NF and RO membranes and additional data are needed. A recent Department of Defense ESTCP pilot scale project (ER20-5369) conducted at Colorado School of Mines (Mines) systematically evaluated the rejection of nine PFAAs by four available NF and RO products using full scale spiral-wound membrane elements in a high recovery membrane system which achieved up to 97% recovery[15]. Tight NF and the two RO membranes evaluated exhibited greater than 98% rejection of all PFAAs evaluated even at high recovery conditions (Figure 3). The loose NF membrane product evaluated provided lower than expected (based on literature) rejection of investigated PFAAs particularly at higher recovery values. These findings indicate that tight NF and RO membranes can be effective at separating PFAAs from contaminated source waters regardless of PFAA chain length. Energy requirements modeled from these experiments varied from 0.14 kWh/m3 for loose NF to 0.57 kWh/m3 for seawater RO[15].
Mines researchers have developed a mobile high-recovery closed-circuit membrane filtration system (Figure 4) that has been successfully deployed for treating groundwater at a fire training area of Wright-Patterson Air Force Base (ESTCP ER21-5136), groundwater at Peterson Space Force Base (AFCEC BAA-031), and firetruck rinsate at Tyndall Air Force Base (ESTCP ER20-5369) during recent ESTCP and AFCEC funded research projects. In these projects, NF or RO was implemented to produce a permeate stream containing low concentrations of PFAS and to concentrate PFAS into smaller volumes of retentate for subsequent destructive PFAS treatment. While NF and RO membranes have demonstrated effective rejection of PFAS, PFAS are subsequently concentrated in the membrane concentrate, or retentate stream. This concentrate stream is increasingly paired with PFAS destruction technologies, as PFAS destruction is often considered viable only for concentrated solutions of PFAS. Ongoing ESTCP funded projects include using high-recovery NF and RO to treat and concentrate groundwater leading to PFAS destruction using plasma based treatment[21] or hydrothermal alkaline treatment (HALT)[22].
Advantages and Limitations of the Technology for PFAS Removal
Advantages:
- Robust, high throughput treatment
- Mature technology with well documented solute separation performance
- High rejection of PFAS and other contaminants
- Removes solutes at the molecular scale
Limitations:
- Complex and often expensive pretreatment requirements for certain waters
- Energy intensive
- High capital costs
- Membrane fouling requiring high chemical usage for cleaning
- Concentrated waste stream requiring disposal or destruction
- Permeate quality depends on feed water concentration
- Greater operation complexity than most water treatment processes
- Water loss due to membrane separation
Summary
High-pressure membranes including NF and RO are well established technologies used in a variety of water treatment fields for the purification of water resources and industrial process waste streams. Research conducted over the past decade has demonstrated that various available membrane products can achieve high rejection of PFAS, enabling compliance with state and federal PFAS regulations. As opposed to adsorbent based PFAS removal technologies (e.g., activated carbon, ion exchange), high-pressure membranes do not have a finite capacity for PFAS removal and do not exhibit breakthrough. High-recovery membrane systems are being implemented into ex situ treatment trains to simultaneously treat PFAS impacted water resources and concentrate PFAS into the retentate stream to enable more effective and efficient PFAS destruction.
References
- ^ 1.0 1.1 Zodrow, J., Arblaster, J., Conder, J., 2021. State of the Science for Risk Assessment of PFAS at Contaminated Sites. In: Forever Chemicals: Environmental, Economic, and Social Equity Concerns with PFAS in the Environment, Kempisty, D., Racz, L., (Ed.s). pp. 161-186. CRC Press. doi: 10.1201/9781003024521
- ^ 2.0 2.1 Conder, J., Zodrow, J., Arblaster, J., Kelly, B., Gobas, F., Suski, J., Osborn, E., Frenchmeyer, M., Divine, C., Leeson, A., 2021. Strategic resources for assessing PFAS ecological risks at AFFF sites. Integrated Environmental Assessment and Management, 17(4), pp. 746-752. doi: 10.1002/ieam.4405
- ^ Lau, C., 2012. Perfluorinated Compounds. In: Molecular, Clinical and Environmental Toxicology, Volume 3: Environmental Toxicology, A. Luch (Ed.), pp. 47-86. Springer Science and Business Media. doi: 10.1007/978-3-7643-8340-4_3
- ^ Field, J., Higgins, C., Deeb, R., Conder, J., 2017. FAQs Regarding PFASs Associated with AFFF Use at U.S. Military Sites. Environmental Security Technology Certification Program (ESTCP) Project ER-201574. Project Website Report.pdf
- ^ Conder, J.M., Hoke, R.A., de Wolf, W., Russell, M.H., Buck, R.C., 2008. Are PFCAs Bioaccumulative? A Critical Review and Comparison with Regulatory Criteria and Persistent Lipophilic Compounds. Environmental Science and Technology, 42(4), pp. 995-1003. doi: 10.1021/es070895g
- ^ 6.0 6.1 United States Environmental Protection Agency (USEPA), 2024. Per- and Polyfluoroalkyl Substances (PFAS) Final PFAS National Primary Drinking Water Regulation. Website
- ^ United States Environmental Protection Agency (USEPA), 2016. Drinking Water Health Advisory for Perfluorooctane Sulfonate (PFOS). Office of Water, EPA 822-R-16-004. Free Download Report.pdf
- ^ United States Environmental Protection Agency (USEPA), 2016b. Drinking Water Health Advisory for Perfluorooctanoic Acid (PFOA). Office of Water, EPA 822-R-16-005. Free Download Report.pdf
- ^ Conder, J., Arblaster, J., Larson, E., Brown, J., Higgins, C., 2020. Guidance for Assessing the Ecological Risks of PFAS to Threatened and Endangered Species at Aqueous Film Forming Foam-Impacted Sites. Strategic Environmental Research and Development Program (SERDP) Project ER 18-1614. Project Website Guidance Document
- ^ Gobas, F.A.P.C., Kelly, B.C., Kim, J.J., 2020. Final Report: A Framework for Assessing Bioaccumulation and Exposure Risks of PFAS in Threatened and Endangered Species on AFFF-Impacted Sites. SERDP Project ER18-1502. Project Website Final Report
- ^ Suski, J.G., 2020. Investigating Potential Risk to Threatened and Endangered Species from Per- and Polyfluoroalkyl Substances (PFAS) on Department of Defense (DoD) Sites. SERDP Project ER18-1626. Project Website Report.pdf
- ^ 12.0 12.1 Zodrow, J.M., Frenchmeyer, M., Dally, K., Osborn, E., Anderson, P. and Divine, C., 2021. Development of Per and Polyfluoroalkyl Substances Ecological Risk-Based Screening Levels. Environmental Toxicology and Chemistry, 40(3), pp. 921-936. doi: 10.1002/etc.4975 Open Access Article
- ^ 13.0 13.1 13.2 Cite error: Invalid
<ref>tag; no text was provided for refs namedApplemanEtAl2013 - ^ 14.0 14.1 Cite error: Invalid
<ref>tag; no text was provided for refs namedSteinle-DarlingReinhard2008 - ^ 15.0 15.1 15.2 Cite error: Invalid
<ref>tag; no text was provided for refs namedSafulkoEtAl2023 - ^ 16.0 16.1 16.2 Liu, C.J., Strathmann, T.J., Bellona, C., 2021. Rejection of per- and polyfluoroalkyl substances (PFASs) in aqueous film-forming foam by high-pressure membranes. Water Research, 188, Article 116546. doi: 10.1016/j.watres.2020.116546
- ^ Wang, J., Wang, L., Xu, C., Zhi, R., Miao, R., Liang, T., Yue, X., Lv, Y., Liu, T., 2018. Perfluorooctane sulfonate and perfluorobutane sulfonate removal from water by nanofiltration membrane: The roles of solute concentration, ionic strength, and macromolecular organic foulants. Chemical Engineering Journal, 332, p. 787-797. doi: 10.1016/j.cej.2017.09.061
- ^ Zhao, C., Tang, C.Y., Li, P., Adrian, P., Hu, G., 2016. Perfluorooctane sulfonate removal by nanofiltration membrane—the effect and interaction of magnesium ion / humic acid. Journal of Membrane Science, 503, p. 31-41. doi: 10.1016/j.memsci.2015.12.049
- ^ Zhao, C., Zhang, J., He, G., Wang, T., Hou, D., Luan, Z., 2013. Perfluorooctane sulfonate removal by nanofiltration membrane the role of calcium ions. Chemical Engineering Journal, 233, p. 224-232. doi: 10.1016/j.cej.2013.08.027
- ^ Steinle-Darling, E., Litwiller, E., Reinhard, M., 2010. Effects of Sorption on the Rejection of Trace Organic Contaminants During Nanofiltration. Environmental Science and Technology, 44(7), p. 2592-2598. doi: 10.1021/es902846m
- ^ Richardson, S., 2021. Nanofiltration Followed by Electrical Discharge Plasma for Destruction of PFAS and Co-occurring Chemicals in Groundwater: A Treatment Train Approach. Environmental Security Technology Certification Program (ESTCP), Project ER21-5136
- ^ Bellona, C., 2023. Cradle to Grave PFAS Treatment Using Membrane and Foam Fractionation Concentration Followed by Hydrothermal Alkaline Treatment. Environmental Security Technology Certification Program (ESTCP), Project ER23-8367