Difference between revisions of "PFAS Ex Situ Water Treatment"
(→Established PFAS Treatment Technologies) |
(Tag: Visual edit) |
||
| (2 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
<onlyinclude>Well-developed ''ex situ'' treatment technologies applicable to treatment of [[Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS) | perfluoroalkyl and polyfluoroalkyl substances (PFAS)]] in drinking water and non-potable groundwater include membrane filtration (reverse osmosis or RO and nanofiltration or NF), activated carbon adsorption (granular and powdered), and anion exchange. However, these technologies are less demonstrated for removal of PFAS from more complex matrices such as wastewater and leachate. There are also a variety of separation and destructive technologies in various stages of development. Some of these processes may also be applicable to more complex matrices including wastewater and landfill leachate. </onlyinclude> | <onlyinclude>Well-developed ''ex situ'' treatment technologies applicable to treatment of [[Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS) | perfluoroalkyl and polyfluoroalkyl substances (PFAS)]] in drinking water and non-potable groundwater include membrane filtration (reverse osmosis or RO and nanofiltration or NF), activated carbon adsorption (granular and powdered), and anion exchange. However, these technologies are less demonstrated for removal of PFAS from more complex matrices such as wastewater and leachate. There are also a variety of separation and destructive technologies in various stages of development. Some of these processes may also be applicable to more complex matrices including wastewater and landfill leachate. </onlyinclude> | ||
<div style="float:right;margin:0 0 2em 2em;">__TOC__</div> | <div style="float:right;margin:0 0 2em 2em;">__TOC__</div> | ||
| + | '''Related Article(s):''' | ||
| − | + | *[[Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS)]] | |
| + | *[[PFAS Soil Remediation Technologies]] | ||
| + | *[[PFAS Sources]] | ||
| + | *[[PFAS Transport and Fate]] | ||
| + | *[[PFAS Treatment by Anion Exchange]] | ||
| + | *[[PFAS Treatment by Electrical Discharge Plasma]] | ||
| + | *[[Photoactivated Reductive Defluorination - PFAS Destruction]] | ||
| + | *[[Supercritical Water Oxidation (SCWO)]] | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
'''Contributor(s):''' [[Dr. Scott Grieco]] and [[James Hatton]] | '''Contributor(s):''' [[Dr. Scott Grieco]] and [[James Hatton]] | ||
| + | |||
'''Key Resource(s):''' | '''Key Resource(s):''' | ||
| − | *[https://www.waterrf.org/resource/treatment-mitigation-strategies-poly-and-perfluorinated-chemicals Water Research Foundation (Drinking Water): Treatment Mitigation Strategies for PFAS]<ref name="Dickenson2016">Dickenson, E. and Higgins, C., 2016. Treatment Mitigation Strategies for Poly- and Perfluoroalkyl Substances, Report Number 4322. Water Research Foundation, Denver, Colorado. 123 pages. ISBN 978-1-60573-234-3</ref> | + | *[https://www.waterrf.org/resource/treatment-mitigation-strategies-poly-and-perfluorinated-chemicals Water Research Foundation (Drinking Water): Treatment Mitigation Strategies for PFAS]<ref name="Dickenson2016">Dickenson, E. and Higgins, C., 2016. Treatment Mitigation Strategies for Poly- and Perfluoroalkyl Substances, Report Number 4322. Water Research Foundation, Denver, Colorado. 123 pages. ISBN 978-1-60573-234-3</ref> |
| − | *[https://pfas-1.itrcweb.org/12-treatment-technologies/#12_2 Interstate Technical and Regulatory Council: PFAS Liquids Treatment Technologies]<ref name="ITRC2020">Interstate Technology and Regulatory Council (ITRC), 2020. PFAS Technical and Regulatory Guidance Document and Fact Sheets, PFAS-1. PFAS Team, Washington, DC. [https://pfas-1.itrcweb.org/ Website] [ | + | *[https://pfas-1.itrcweb.org/12-treatment-technologies/#12_2 Interstate Technical and Regulatory Council: PFAS Liquids Treatment Technologies]<ref name="ITRC2020">Interstate Technology and Regulatory Council (ITRC), 2020. PFAS Technical and Regulatory Guidance Document and Fact Sheets, PFAS-1. PFAS Team, Washington, DC. [https://pfas-1.itrcweb.org/ Website] [//www.enviro.wiki/images/2/2e/ITRC_PFAS-1.pdf Report.pdf]</ref> |
*[https://www.sciencedirect.com/science/article/pii/S0301479717307934 Novel treatment technologies for PFAS compounds: A critical review.]<ref name="Kucharzyk2017">Kucharzyk, K.H., Darlington, R., Benotti, M., Deeb, R. and Hawley, E., 2017. Novel treatment technologies for PFAS compounds: A critical review. Journal of Environmental Management, 204(2), pp. 757-764. [https://doi.org/10.1016/j.jenvman.2017.08.016 DOI: 10.1016/j.jenvman.2017.08.016] Manuscript available from: [https://www.researchgate.net/profile/Katarzyna_kate_Kucharzyk/publication/319125507_Novel_treatment_technologies_for_PFAS_compounds_A_critical_review/links/5a06590b4585157013a3be77/Novel-treatment-technologies-for-PFAS-compounds-A-critical-review.pdf ResearchGate].</ref> | *[https://www.sciencedirect.com/science/article/pii/S0301479717307934 Novel treatment technologies for PFAS compounds: A critical review.]<ref name="Kucharzyk2017">Kucharzyk, K.H., Darlington, R., Benotti, M., Deeb, R. and Hawley, E., 2017. Novel treatment technologies for PFAS compounds: A critical review. Journal of Environmental Management, 204(2), pp. 757-764. [https://doi.org/10.1016/j.jenvman.2017.08.016 DOI: 10.1016/j.jenvman.2017.08.016] Manuscript available from: [https://www.researchgate.net/profile/Katarzyna_kate_Kucharzyk/publication/319125507_Novel_treatment_technologies_for_PFAS_compounds_A_critical_review/links/5a06590b4585157013a3be77/Novel-treatment-technologies-for-PFAS-compounds-A-critical-review.pdf ResearchGate].</ref> | ||
| Line 25: | Line 29: | ||
Three technologies are well demonstrated for removal of [[Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS) | PFAS]] from drinking water and non-potable groundwater (as described below): | Three technologies are well demonstrated for removal of [[Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS) | PFAS]] from drinking water and non-potable groundwater (as described below): | ||
| − | * membrane filtration including [[wikipedia: Reverse osmosis | reverse osmosis (RO)]] and [[Wikipedia: Nanofiltration | nanofiltration (NF)]] | + | *membrane filtration including [[wikipedia: Reverse osmosis | reverse osmosis (RO)]] and [[Wikipedia: Nanofiltration | nanofiltration (NF)]] |
| − | * [[Wikipedia: Activated_carbon#Classification | granular activated carbon (GAC) and powdered activated carbon (PAC)]] adsorption | + | *[[Wikipedia: Activated_carbon#Classification | granular activated carbon (GAC) and powdered activated carbon (PAC)]] adsorption |
| − | * [[wikipedia: Ion_exchange | anion exchange (IX)]] | + | *[[wikipedia: Ion_exchange | anion exchange (IX)]] |
| − | However, these technologies are less demonstrated for removal of PFAS from more complex matrices such as wastewater and leachate. <onlyinclude>Site-specific considerations that affect the selection of optimum treatment technologies for a given site include water chemistry, required flow rate, treatment criteria, waste residual generation, residual disposal options, and operational complexity. Treatability studies with site water are highly recommended because every site has different factors that may affect engineering design for these technologies. | + | However, these technologies are less demonstrated for removal of PFAS from more complex matrices such as wastewater and leachate. <onlyinclude>Site-specific considerations that affect the selection of optimum treatment technologies for a given site include water chemistry, required flow rate, treatment criteria, waste residual generation, residual disposal options, and operational complexity. Treatability studies with site water are highly recommended because every site has different factors that may affect engineering design for these technologies. |
</onlyinclude> | </onlyinclude> | ||
===Membrane Filtration=== | ===Membrane Filtration=== | ||
[[File: revOsmosisPlant.png | thumb | 500px | Figure 1. A RO municipal drinking water plant in Arizona]] | [[File: revOsmosisPlant.png | thumb | 500px | Figure 1. A RO municipal drinking water plant in Arizona]] | ||
| − | <onlyinclude>Given their ability to remove dissolved contaminants at a molecular size level, RO and some NF membranes can be highly effective for PFAS removal. For RO systems</onlyinclude> (Figure 1)<onlyinclude>, several studies have demonstrated effective removal of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS)</onlyinclude> (see [[Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS) | PFAS]] for nomenclature)<onlyinclude> from drinking water with removal rates well above 90%</onlyinclude><ref name="Tang2006">Tang, C.Y., Fu, Q.S., Robertson, A.P., Criddle, C.S., and Leckie, J.O., 2006. Use of Reverse Osmosis Membranes to Remove Perfluorooctane Sulfonate (PFOS) from Semiconductor Wastewater. Environmental Science and Technology, 40(23), pp. 7343-7349. [https://doi.org/10.1021/es060831q DOI: 10.1021/es060831q]</ref><ref name="Flores2013">Flores, C., Ventura, F., Martin-Alonso, J., and Caixach, J., 2013. Occurrence of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in NE Spanish surface waters and their removal in a drinking water treatment plant that combines conventional and advanced treatments in parallel lines. Science of the Total environment, 461, 618-626. [https://doi.org/10.1016/j.scitotenv.2013.05.026 DOI: 10.1016/j.scitotenv.2013.05.026]</ref><ref name="Appleman2014">Appleman, T.D., Higgins, C.P., Quiñones, O., Vanderford, B.J., Kolstad, C., Zeigler-Holady, J.C., and Dickenson, E.R., 2014. Treatment of poly- and perfluoroalkyl substances in US full-scale water treatment systems. Water Research, 51, pp. 246-255. [https://doi.org/10.1016/j.watres.2013.10.067 DOI: 10.1016/j.watres.2013.10.067]</ref>. RO potable water reuse treatment systems implemented in California have also demonstrated effective PFOS and PFOA removal as reported by the Water Research Foundation (WRF)<ref name="Dickenson2016"/>. Analysis of permeate at both sites referenced by the WRF confirmed that short and long chain PFAS concentrations in the treated water were reduced to levels below test method reporting limits<onlyinclude>.</onlyinclude> | + | <onlyinclude>Given their ability to remove dissolved contaminants at a molecular size level, RO and some NF membranes can be highly effective for PFAS removal. For RO systems</onlyinclude> (Figure 1)<onlyinclude>, several studies have demonstrated effective removal of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS)</onlyinclude> (see [[Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS) | PFAS]] for nomenclature)<onlyinclude> from drinking water with removal rates well above 90%</onlyinclude><ref name="Tang2006">Tang, C.Y., Fu, Q.S., Robertson, A.P., Criddle, C.S., and Leckie, J.O., 2006. Use of Reverse Osmosis Membranes to Remove Perfluorooctane Sulfonate (PFOS) from Semiconductor Wastewater. Environmental Science and Technology, 40(23), pp. 7343-7349. [https://doi.org/10.1021/es060831q DOI: 10.1021/es060831q]</ref><ref name="Flores2013">Flores, C., Ventura, F., Martin-Alonso, J., and Caixach, J., 2013. Occurrence of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in NE Spanish surface waters and their removal in a drinking water treatment plant that combines conventional and advanced treatments in parallel lines. Science of the Total environment, 461, 618-626. [https://doi.org/10.1016/j.scitotenv.2013.05.026 DOI: 10.1016/j.scitotenv.2013.05.026]</ref><ref name="Appleman2014">Appleman, T.D., Higgins, C.P., Quiñones, O., Vanderford, B.J., Kolstad, C., Zeigler-Holady, J.C., and Dickenson, E.R., 2014. Treatment of poly- and perfluoroalkyl substances in US full-scale water treatment systems. Water Research, 51, pp. 246-255. [https://doi.org/10.1016/j.watres.2013.10.067 DOI: 10.1016/j.watres.2013.10.067]</ref>. RO potable water reuse treatment systems implemented in California have also demonstrated effective PFOS and PFOA removal as reported by the Water Research Foundation (WRF)<ref name="Dickenson2016" />. Analysis of permeate at both sites referenced by the WRF confirmed that short and long chain PFAS concentrations in the treated water were reduced to levels below test method reporting limits<onlyinclude>.</onlyinclude> |
| − | Full-scale studies using larger effective pore size NF membranes for PFAS removal are limited in number but are promising since NF systems are somewhat less costly than RO and may be nearly as effective in removing PFAS. Recent laboratory or pilot studies have shown good performance of NF membranes<ref name="Steinle-Darling2008">Steinle-Darling, E., and Reinhard, M., 2008. Nanofiltration for Trace Organic Contaminant Removal: Structure, Solution, and Membrane Fouling Effects on the Rejection of Perfluorochemicals. Environmental Science and Technology, 42(14), pp. 5292-5297. [https://doi.org/10.1021/es703207s DOI: 10.1021/es703207s] Free download from: [https://d1wqtxts1xzle7.cloudfront.net/48926882/es703207s20160918-21142-1xmqco5.pdf?1474189169=&response-content-disposition=inline%3B+filename%3DNanofiltration_for_Trace_Organic_Contami.pdf&Expires=1613000850&Signature=N-ZvvjOJX3TSOQzg7od3Q0LulNSZOqqjfummVEUfmiYlC3VasS4FuBHOgY52Xy~7FrKbOLhx0xx8QHdUsR~fbRTMQNXhiqbEslnU2gda2EcZHMMJj0mf-01wIA3jFIywA7IIabmTd3uMUGsIfT1D0PrGY00RmprYIQBoG3Dg~KjoizdfxYfvEgdZw2C~7D47pPiwMSnavZiGuvO0~dbRF8nawL7Prg91xt5BFTNUQQiIrIlMWc4PhVjzE5Su2CUZqnNlYdAW5Ck7B9lKmmVMPiOgz07vFnyp7m-q4UK3woa~aBFW9Wp~hjqN6vfohn8Hocv5oMpZNamhu8vBbPilKw__&Key-Pair-Id=APKAJLOHF5GGSLRBV4ZA Academia].</ref><ref name="Appleman2013">Appleman, T.D., Dickenson, E.R., Bellona, C., and Higgins, C.P., 2013. Nanofiltration and granular activated carbon treatment of perfluoroalkyl acids. Journal of Hazardous Materials, 260, 740-746. [https://doi.org/10.1016/j.jhazmat.2013.06.033 DOI: 10.1016/j.jhazmat.2013.06.033]</ref><ref name="Soriano2017">Soriano, Á., Gorri, D., and Urtiaga, A., 2017. Efficient treatment of perfluorohexanoic acid by nanofiltration followed by electrochemical degradation of the NF concentrate. Water Research, 112, 147-156. [https://doi.org/10.1016/j.watres.2017.01.043 DOI: 10.1016/j.watres.2017.01.043] [ | + | Full-scale studies using larger effective pore size NF membranes for PFAS removal are limited in number but are promising since NF systems are somewhat less costly than RO and may be nearly as effective in removing PFAS. Recent laboratory or pilot studies have shown good performance of NF membranes<ref name="Steinle-Darling2008">Steinle-Darling, E., and Reinhard, M., 2008. Nanofiltration for Trace Organic Contaminant Removal: Structure, Solution, and Membrane Fouling Effects on the Rejection of Perfluorochemicals. Environmental Science and Technology, 42(14), pp. 5292-5297. [https://doi.org/10.1021/es703207s DOI: 10.1021/es703207s] Free download from: [https://d1wqtxts1xzle7.cloudfront.net/48926882/es703207s20160918-21142-1xmqco5.pdf?1474189169=&response-content-disposition=inline%3B+filename%3DNanofiltration_for_Trace_Organic_Contami.pdf&Expires=1613000850&Signature=N-ZvvjOJX3TSOQzg7od3Q0LulNSZOqqjfummVEUfmiYlC3VasS4FuBHOgY52Xy~7FrKbOLhx0xx8QHdUsR~fbRTMQNXhiqbEslnU2gda2EcZHMMJj0mf-01wIA3jFIywA7IIabmTd3uMUGsIfT1D0PrGY00RmprYIQBoG3Dg~KjoizdfxYfvEgdZw2C~7D47pPiwMSnavZiGuvO0~dbRF8nawL7Prg91xt5BFTNUQQiIrIlMWc4PhVjzE5Su2CUZqnNlYdAW5Ck7B9lKmmVMPiOgz07vFnyp7m-q4UK3woa~aBFW9Wp~hjqN6vfohn8Hocv5oMpZNamhu8vBbPilKw__&Key-Pair-Id=APKAJLOHF5GGSLRBV4ZA Academia].</ref><ref name="Appleman2013">Appleman, T.D., Dickenson, E.R., Bellona, C., and Higgins, C.P., 2013. Nanofiltration and granular activated carbon treatment of perfluoroalkyl acids. Journal of Hazardous Materials, 260, 740-746. [https://doi.org/10.1016/j.jhazmat.2013.06.033 DOI: 10.1016/j.jhazmat.2013.06.033]</ref><ref name="Soriano2017">Soriano, Á., Gorri, D., and Urtiaga, A., 2017. Efficient treatment of perfluorohexanoic acid by nanofiltration followed by electrochemical degradation of the NF concentrate. Water Research, 112, 147-156. [https://doi.org/10.1016/j.watres.2017.01.043 DOI: 10.1016/j.watres.2017.01.043] [//www.enviro.wiki/images/4/47/Soriano2017.pdf Author’s Manuscript.]</ref><ref name="Zeng2017">Zeng, C., Tanaka, S., Suzuki, Y., Yukioka, S., and Fujii, S., 2017. Rejection of Trace Level Perfluorohexanoic Acid (PFHxA) in Pure Water by Loose Nanofiltration Membrane. Journal of Water and Environment Technology, 15(3), pp. 120-127. [https://doi.org/10.2965/jwet.16-072 DOI: 10.2965/jwet.16-072] Free download from: [https://www.jstage.jst.go.jp/article/jwet/15/3/15_16-072/_pdf J-STAGE]</ref><ref name="Wang2018">Wang, J., Wang, L., Xu, C., Zhi, R., Miao, R., Liang, T., Yue, X., Lv, Y. and Liu, T., 2018. Perfluorooctane sulfonate and perfluorobutane sulfonate removal from water by nanofiltration membrane: The roles of solute concentration, ionic strength, and macromolecular organic foulants. Chemical Engineering Journal, 332, pp. 787-797. [https://doi.org/10.1016/j.cej.2017.09.061 DOI: 10.1016/j.cej.2017.09.061]</ref>. |
Although membrane RO and NF processes are generally capable of providing uniform removal rates relative to short and long chain PFAS compounds (see [[Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS) | PFAS]] for nomenclature), other aspects of these treatment technologies are more challenging: | Although membrane RO and NF processes are generally capable of providing uniform removal rates relative to short and long chain PFAS compounds (see [[Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS) | PFAS]] for nomenclature), other aspects of these treatment technologies are more challenging: | ||
| − | * Membranes must be flushed and cleaned periodically, such that overall water recovery rates (process water volumes consumed, wasted, and lost vs. treated water volumes produced) are much lower than those for GAC and IX processes. Membrane fouling can be slowed or avoided depending on operating conditions, membrane modifications, and feed modifications<ref name="LeRoux2005">Le Roux, I., Krieg, H.M., Yeates, C.A. and Breytenbach, J.C., 2005. Use of chitosan as an antifouling agent in a membrane bioreactor. Journal of Membrane Science, 248(1-2), pp. 127-136. [https://doi.org/10.1016/j.memsci.2004.10.005 DOI: 10.1016/j.memsci.2004.10.005]</ref>. Typically, 70-90% of the water supplied into a membrane RO process is recoverable as treated water. The remaining 10-30% is reject containing approximately 4 to 8 times the initial PFAS concentration (depending on recovery rate). | + | *Membranes must be flushed and cleaned periodically, such that overall water recovery rates (process water volumes consumed, wasted, and lost vs. treated water volumes produced) are much lower than those for GAC and IX processes. Membrane fouling can be slowed or avoided depending on operating conditions, membrane modifications, and feed modifications<ref name="LeRoux2005">Le Roux, I., Krieg, H.M., Yeates, C.A. and Breytenbach, J.C., 2005. Use of chitosan as an antifouling agent in a membrane bioreactor. Journal of Membrane Science, 248(1-2), pp. 127-136. [https://doi.org/10.1016/j.memsci.2004.10.005 DOI: 10.1016/j.memsci.2004.10.005]</ref>. Typically, 70-90% of the water supplied into a membrane RO process is recoverable as treated water. The remaining 10-30% is reject containing approximately 4 to 8 times the initial PFAS concentration (depending on recovery rate). |
| − | * These cleaning and flushing processes create a continuous liquid waste stream, which periodically includes harsh membrane cleaning chemicals as well as a continuous flow of concentrated membrane reject chemicals (i.e., PFAS) that must be properly managed and disposed of. Management often includes further treatment to remove PFAS from the liquid waste. | + | *These cleaning and flushing processes create a continuous liquid waste stream, which periodically includes harsh membrane cleaning chemicals as well as a continuous flow of concentrated membrane reject chemicals (i.e., PFAS) that must be properly managed and disposed of. Management often includes further treatment to remove PFAS from the liquid waste. |
| − | * RO and NF systems are inherently more expensive and complicated systems to implement, operate, and maintain compared to adsorption processes. Treatment system operator certification and process monitoring requirements are correspondingly markedly higher for RO and NF than they are for GAC and IX. | + | *RO and NF systems are inherently more expensive and complicated systems to implement, operate, and maintain compared to adsorption processes. Treatment system operator certification and process monitoring requirements are correspondingly markedly higher for RO and NF than they are for GAC and IX. |
| − | * Water feed pressures required to drive flow through membrane RO and NF processes are considerably higher than those involved with GAC and IX processes. This results in reduced process efficiency and higher pumping and electrical operating costs. | + | *Water feed pressures required to drive flow through membrane RO and NF processes are considerably higher than those involved with GAC and IX processes. This results in reduced process efficiency and higher pumping and electrical operating costs. |
| − | * Membrane systems can also be subject to issues with irreversible membrane fouling, clogging, and scaling or other physical membrane damage and failures. Additional water pretreatment and higher levels of monitoring and maintenance are then required, further adding to the higher costs of such systems. | + | *Membrane systems can also be subject to issues with irreversible membrane fouling, clogging, and scaling or other physical membrane damage and failures. Additional water pretreatment and higher levels of monitoring and maintenance are then required, further adding to the higher costs of such systems. |
===Activated Carbon Adsorption=== | ===Activated Carbon Adsorption=== | ||
| Line 55: | Line 59: | ||
<onlyinclude>The removal efficiency of individual PFAS compounds using GAC is a function of both the PFAS functional group (carboxylic acid versus sulfonic acid) and also the perfluoro-carbon chain length</onlyinclude><ref name="McCleaf2017">McCleaf, P., Englund, S., Östlund, A., Lindegren, K., Wiberg, K., and Ahrens, L., 2017. Removal efficiency of multiple poly-and perfluoroalkyl substances (PFASs) in drinking water using granular activated carbon (GAC) and anion exchange (AE) column tests. Water Research, 120, pp. 77-87. [https://doi.org/10.1016/j.watres.2017.04.057 DOI: 10.1016/j.watres.2017.04.057]</ref><ref name="Eschauzier2012">Eschauzier, C., Beerendonk, E., Scholte-Veenendaal, P., and De Voogt, P., 2012. Impact of Treatment Processes on the Removal of Perfluoroalkyl Acids from the Drinking Water Production Chain. Environmental Science and Technology, 46(3), pp. 1708-1715. [https://doi.org/10.1021/es201662b DOI: 10.1021/es201662b]</ref>(see [[Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS) | PFAS]] for nomenclature): | <onlyinclude>The removal efficiency of individual PFAS compounds using GAC is a function of both the PFAS functional group (carboxylic acid versus sulfonic acid) and also the perfluoro-carbon chain length</onlyinclude><ref name="McCleaf2017">McCleaf, P., Englund, S., Östlund, A., Lindegren, K., Wiberg, K., and Ahrens, L., 2017. Removal efficiency of multiple poly-and perfluoroalkyl substances (PFASs) in drinking water using granular activated carbon (GAC) and anion exchange (AE) column tests. Water Research, 120, pp. 77-87. [https://doi.org/10.1016/j.watres.2017.04.057 DOI: 10.1016/j.watres.2017.04.057]</ref><ref name="Eschauzier2012">Eschauzier, C., Beerendonk, E., Scholte-Veenendaal, P., and De Voogt, P., 2012. Impact of Treatment Processes on the Removal of Perfluoroalkyl Acids from the Drinking Water Production Chain. Environmental Science and Technology, 46(3), pp. 1708-1715. [https://doi.org/10.1021/es201662b DOI: 10.1021/es201662b]</ref>(see [[Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS) | PFAS]] for nomenclature): | ||
| − | * perfluoro-sulfonate acids (PFSAs) are more efficiently removed than perfluoro-carboxylic acids (PFCAs) of the same chain length | + | |
| − | * long chain compounds of the same functional group are removed better than the shorter chains | + | *perfluoro-sulfonate acids (PFSAs) are more efficiently removed than perfluoro-carboxylic acids (PFCAs) of the same chain length |
| − | Activated carbon may be applied in drinking water systems as GAC or PAC<ref name="Dudley">Dudley, L.A., Arevalo, E.C., and Knappe, D.R., 2015. Removal of Perfluoroalkyl Substances by PAC Adsorption and Anion Exchange. Water Research Foundation Project #4344. Free download of Executive Summary from: [https://www.waterrf.org/system/files/resource/2019-04/4344_ProjectSummary.pdf Water Research Foundation (Public Plus account)]</ref><ref name="Qian2017">Qian, J., Shen, M., Wang, P., Wang, C., Li, K., Liu, J., Lu, B. and Tian, X., 2017. Perfluorooctane sulfonate adsorption on powder activated carbon: Effect of phosphate (P) competition, pH, and temperature. Chemosphere, 182, pp. 215-222. [https://doi.org/10.1016/j.chemosphere.2017.05.033 DOI: 10.1016/j.chemosphere.2017.05.033]</ref>. GAC has larger granules and is reusable, while PAC has much smaller granules and is not typically reused. PAC has most often been used as a temporary treatment because costs associated with disposal and replacement of the used PAC tend to preclude using it for long-term treatment. A typical GAC installation for a private drinking water well is shown in Figure 2. Contrary to PAC, GAC used to treat PFAS can be reactivated by the manufacturer, driving the PFAS from the GAC and into off-gas. The extracted gas is then treated with thermal oxidation (temperatures often 1200°C to 1400°C). The reactivated GAC is then brought back to the site and reused. Thus, GAC can ultimately be a destructive treatment technology<onlyinclude>. </onlyinclude> | + | *long chain compounds of the same functional group are removed better than the shorter chains |
| + | |||
| + | Activated carbon may be applied in drinking water systems as GAC or PAC<ref name="Dudley">Dudley, L.A., Arevalo, E.C., and Knappe, D.R., 2015. Removal of Perfluoroalkyl Substances by PAC Adsorption and Anion Exchange. Water Research Foundation Project #4344. Free download of Executive Summary from: [https://www.waterrf.org/system/files/resource/2019-04/4344_ProjectSummary.pdf Water Research Foundation (Public Plus account)]</ref><ref name="Qian2017">Qian, J., Shen, M., Wang, P., Wang, C., Li, K., Liu, J., Lu, B. and Tian, X., 2017. Perfluorooctane sulfonate adsorption on powder activated carbon: Effect of phosphate (P) competition, pH, and temperature. Chemosphere, 182, pp. 215-222. [https://doi.org/10.1016/j.chemosphere.2017.05.033 DOI: 10.1016/j.chemosphere.2017.05.033]</ref>. GAC has larger granules and is reusable, while PAC has much smaller granules and is not typically reused. PAC has most often been used as a temporary treatment because costs associated with disposal and replacement of the used PAC tend to preclude using it for long-term treatment. A typical GAC installation for a private drinking water well is shown in Figure 2. Contrary to PAC, GAC used to treat PFAS can be reactivated by the manufacturer, driving the PFAS from the GAC and into off-gas. The extracted gas is then treated with thermal oxidation (temperatures often 1200°C to 1400°C). The reactivated GAC is then brought back to the site and reused. Thus, GAC can ultimately be a destructive treatment technology<onlyinclude>.</onlyinclude> | ||
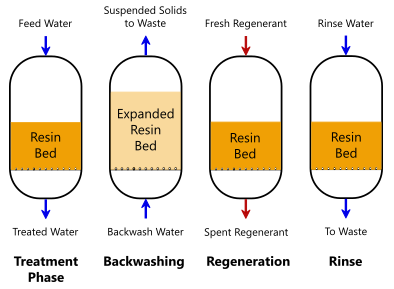
[[File: IXcycle.png | thumb | 400px | left | Figure 3. Operational cycle of a packed bed reactor with anion exchange resin beads]] | [[File: IXcycle.png | thumb | 400px | left | Figure 3. Operational cycle of a packed bed reactor with anion exchange resin beads]] | ||
===Anion Exchange=== | ===Anion Exchange=== | ||
| − | <onlyinclude>Anion exchange has also been demonstrated for the adsorption of PFAS, and published results note higher sorption per pound than GAC</onlyinclude><ref name="McCleaf2017"/><ref name=" Senevirathna2010">Senevirathna, S.T.M.L.D., Tanaka, S., Fujii, S., Kunacheva, C., Harada, H., Shivakoti, B.R., and Okamoto, R., 2010. A comparative study of adsorption of perfluorooctane sulfonate (PFOS) onto granular activated carbon, ion-exchange polymers and non-ion-exchange polymers. Chemosphere, 80(6), pp. 647-651. [https://doi.org/10.1016/j.chemosphere.2010.04.053 DOI: 10.1016/j.chemosphere.2010.04.053] Free download from: [https://www.researchgate.net/profile/Chinagarn_Kunacheva/publication/44672056_A_comparative_study_of_adsorption_of_perfluorooctane_sulfonate_PFOS_onto_granular_activated_carbon_ion-exchange_polymers_and_non-ion-exchange_polymers/links/5a3380510f7e9b2a288a2b21/A-comparative-study-of-adsorption-of-perfluorooctane-sulfonate-PFOS-onto-granular-activated-carbon-ion-exchange-polymers-and-non-ion-exchange-polymers.pdf ResearchGate]</ref><ref name="Woodard2017">Woodard, S., Berry, J., and Newman, B., 2017. Ion exchange resin for PFAS removal and pilot test comparison to GAC. Remediation Journal, 27(3), pp. 19-27. [https://doi.org/10.1002/rem.21515 DOI: 10.1002/rem.21515]</ref>. The higher capacity is believed to be due to combined hydrophobic and ion exchange adsorption mechanisms, whereas GAC mainly relies on hydrophobic attraction. Anion exchange resins can be highly selective, or they can also remove other contaminants based on design requirements and water chemistry. Resins have greater affinity for PFAS subgroup PFSA than for PFCA, and affinity increases with carbon chain length. | + | <onlyinclude>Anion exchange has also been demonstrated for the adsorption of PFAS, and published results note higher sorption per pound than GAC</onlyinclude><ref name="McCleaf2017" /><ref name="Senevirathna2010">Senevirathna, S.T.M.L.D., Tanaka, S., Fujii, S., Kunacheva, C., Harada, H., Shivakoti, B.R., and Okamoto, R., 2010. A comparative study of adsorption of perfluorooctane sulfonate (PFOS) onto granular activated carbon, ion-exchange polymers and non-ion-exchange polymers. Chemosphere, 80(6), pp. 647-651. [https://doi.org/10.1016/j.chemosphere.2010.04.053 DOI: 10.1016/j.chemosphere.2010.04.053] Free download from: [https://www.researchgate.net/profile/Chinagarn_Kunacheva/publication/44672056_A_comparative_study_of_adsorption_of_perfluorooctane_sulfonate_PFOS_onto_granular_activated_carbon_ion-exchange_polymers_and_non-ion-exchange_polymers/links/5a3380510f7e9b2a288a2b21/A-comparative-study-of-adsorption-of-perfluorooctane-sulfonate-PFOS-onto-granular-activated-carbon-ion-exchange-polymers-and-non-ion-exchange-polymers.pdf ResearchGate]</ref><ref name="Woodard2017">Woodard, S., Berry, J., and Newman, B., 2017. Ion exchange resin for PFAS removal and pilot test comparison to GAC. Remediation Journal, 27(3), pp. 19-27. [https://doi.org/10.1002/rem.21515 DOI: 10.1002/rem.21515]</ref>. The higher capacity is believed to be due to combined hydrophobic and ion exchange adsorption mechanisms, whereas GAC mainly relies on hydrophobic attraction. Anion exchange resins can be highly selective, or they can also remove other contaminants based on design requirements and water chemistry. Resins have greater affinity for PFAS subgroup PFSA than for PFCA, and affinity increases with carbon chain length. |
| − | [[Wikipedia: Ion-exchange resin | Anion exchange resins]] are a viable alternative to GAC for ''ex situ'' treatment of PFAS anions, and several venders sell resins capable of removing PFAS. Resins available for treating PFAS include regenerable resins that can be used multiple times (Figure 3) and single-use resins that must be disposed or destroyed after use<ref name=" Senevirathna2010"/>. Regenerable resins generate a solvent and brine solution, which is distilled to recover the solvent prior to the brine being adsorbed onto a small quantity of GAC or resin for ultimate disposal. This use of one treatment technology (GAC, IX) to support another (RO) is sometimes referred to as a “treatment train” approach. Single-use resins can be more fully exhausted than regenerable resins can and may be a more cost-effective solution for low concentration PFAS contamination, while regenerable resins may be more cost effective for higher concentration contamination<onlyinclude>. </onlyinclude> | + | [[Wikipedia: Ion-exchange resin | Anion exchange resins]] are a viable alternative to GAC for ''ex situ'' treatment of PFAS anions, and several venders sell resins capable of removing PFAS. Resins available for treating PFAS include regenerable resins that can be used multiple times (Figure 3) and single-use resins that must be disposed or destroyed after use<ref name="Senevirathna2010" />. Regenerable resins generate a solvent and brine solution, which is distilled to recover the solvent prior to the brine being adsorbed onto a small quantity of GAC or resin for ultimate disposal. This use of one treatment technology (GAC, IX) to support another (RO) is sometimes referred to as a “treatment train” approach. Single-use resins can be more fully exhausted than regenerable resins can and may be a more cost-effective solution for low concentration PFAS contamination, while regenerable resins may be more cost effective for higher concentration contamination<onlyinclude>.</onlyinclude> |
==Developing PFAS Treatment Technologies== | ==Developing PFAS Treatment Technologies== | ||
{| class="wikitable" style="float:right; margin-left:10px;" | {| class="wikitable" style="float:right; margin-left:10px;" | ||
| − | |+ Table 1. Developmental Technologies | + | |+Table 1. Developmental Technologies |
|- | |- | ||
| − | ! Stage | + | !Stage |
| − | ! Separation/Transfer | + | !Separation/Transfer |
| − | ! Destructive* | + | !Destructive* |
|- | |- | ||
| − | | Developing | + | |Developing |
| + | | | ||
| + | *Biochar<ref name="Guo2017">Guo, W., Huo, S., Feng, J., and Lu, X., 2017. Adsorption of perfluorooctane sulfonate (PFOS) on corn straw-derived biochar prepared at different pyrolytic temperatures. Journal of the Taiwan Institute of Chemical Engineers, 78, pp. 265-271. [https://doi.org/10.1016/j.jtice.2017.06.013 DOI: 10.1016/j.jtice.2017.06.013]</ref><ref name="Kupryianchyk2016">Kupryianchyk, D., Hale, S.E., Breedveld, G.D., and Cornelissen, G., 2016. Treatment of sites contaminated with perfluorinated compounds using biochar amendment. Chemosphere, 142, pp. 35-40. [https://doi.org/10.1016/j.chemosphere.2015.04.085 DOI: 10.1016/j.chemosphere.2015.04.085] Free download from: [https://www.researchgate.net/profile/Sarah_Hale3/publication/276067521_Treatment_of_sites_contaminated_with_perfluorinated_compounds_using_biochar_amendment/links/5cdbe03b299bf14d959895d9/Treatment-of-sites-contaminated-with-perfluorinated-compounds-using-biochar-amendment.pdf ResearchGate]</ref><ref name="Inyang2017">Inyang, M., and Dickenson, E.R., 2017. The use of carbon adsorbents for the removal of perfluoroalkyl acids from potable reuse systems. Chemosphere, 184, pp. 168-175. [https://doi.org/10.1016/j.chemosphere.2017.05.161 DOI: 10.1016/j.chemosphere.2017.05.161]</ref> | ||
| + | *Modified Zeolites<ref name="Espana2015">Espana, V.A.A., Mallavarapu, M., and Naidu, R., 2015. Treatment technologies for aqueous perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA): A critical review with an emphasis on field testing. Environmental Technology and Innovation, 4, pp. 168-181. [https://doi.org/10.1016/j.eti.2015.06.001 DOI: 10.1016/j.eti.2015.06.001] Free download from: [https://www.researchgate.net/profile/Ravi_Naidu2/publication/341241612_Recent_advances_in_the_analysis_of_per-and_polyfluoroalkyl_substances_PFAS-A_review/links/5eb9e3d892851cd50dab441c/Recent-advances-in-the-analysis-of-per-and-polyfluoroalkyl-substances-PFAS-A-review.pdf ResearchGate]</ref><ref name="CETCO2019">CETCO, 2019. FLUORO-SORB® Adsorbent (product sales brochure). [https://www.mineralstech.com/docs/default-source/performance-materials-documents/cetco/environmental-products/brochures/ps_fluorosorb_am_en_201905_v1.pdf Free download] [//www.enviro.wiki/images/4/4f/FluoroSorb2019.pdf Fluoro-Sorb.pdf]</ref> | ||
| + | *Specialty adsorbents<ref name="Zhang2011">Zhang, Q., Deng, S., Yu, G., and Huang, J., 2011. Removal of perfluorooctane sulfonate from aqueous solution by crosslinked chitosan beads: sorption kinetics and uptake mechanism. Bioresource Technology, 102(3), pp. 2265-2271. [https://doi.org/10.1016/j.biortech.2010.10.040 DOI: 10.1016/j.biortech.2010.10.040]</ref><ref name="Cao2016">Cao, F., Wang, L., Ren, X., and Sun, H., 2016. Synthesis of a perfluorooctanoic acid molecularly imprinted polymer for the selective removal of perfluorooctanoic acid in an aqueous environment. Journal of Applied Polymer Science, 133(15). [https://doi.org/10.1002/app.43192 DOI: 10.1002/app.43192]</ref><ref name="Hu2016">Hu, L., Li, Y., and Zhang, W., 2016. Characterization and application of surface-molecular-imprinted-polymer modified TiO2 nanotubes for removal of perfluorinated chemicals. Water Science and Technology, 74(6), pp. 1417-1425. [https://doi.org/10.2166/wst.2016.321 DOI: 10.2166/wst.2016.321] [//www.enviro.wiki/images/0/07/Hu2016.pdf Free access article.]</ref> | ||
| | | | ||
| − | + | *Electro-oxidation<ref name="Zhang2016">Zhang, C., Tang, J., Peng, C., and Jin, M., 2016. Degradation of perfluorinated compounds in wastewater treatment plant effluents by electrochemical oxidation with Nano-ZnO coated electrodes. Journal of Molecular Liquids, 221, pp. 1145-1150. [https://doi.org/10.1016/j.molliq.2016.06.093 DOI: 10.1016/j.molliq.2016.06.093]</ref><ref name="Urtiaga2015">Urtiaga, A., Fernández-González, C., Gómez-Lavín, S., and Ortiz, I., 2015. Kinetics of the electrochemical mineralization of perfluorooctanoic acid on ultrananocrystalline boron doped conductive diamond electrodes. Chemosphere, 129, pp. 20-26. [https://doi.org/10.1016/j.chemosphere.2014.05.090 DOI: 10.1016/j.chemosphere.2014.05.090]</ref><ref name="Schaefer2018">Schaefer, C.E., Choyke, S., Ferguson, P.L., Andaya, C., Burant, A., Maizel, A., Strathmann, T.J. and Higgins, C.P., 2018. Electrochemical Transformations of Perfluoroalkyl Acid (PFAA) Precursors and PFAAs in Groundwater Impacted with Aqueous Film Forming Foams. Environmental Science and Technology, 52(18), pp. 10689-10697. [https://doi.org/10.1021/acs.est.8b02726 DOI: 10.1021/acs.est.8b02726]</ref> | |
| − | + | *Heat activated persulfate<ref name="Park2016">Park, S., Lee, L.S., Medina, V. F., Zull, A., and Waisner, S., 2016. Heat-activated persulfate oxidation of PFOA, 6: 2 fluorotelomer sulfonate, and PFOS under conditions suitable for in-situ groundwater remediation. Chemosphere, 145, pp. 376-383. [https://doi.org/10.1016/j.chemosphere.2015.11.097 DOI: 10.1016/j.chemosphere.2015.11.097]</ref> | |
| − | + | *Alkaline peroxone<ref name="Lin2012">Lin, A.Y.C., Panchangam, S.C., Chang, C.Y., Hong, P.A., and Hsueh, H.F., 2012. Removal of perfluorooctanoic acid and perfluorooctane sulfonate via ozonation under alkaline condition. Journal of Hazardous Materials, 243, pp. 272-277. [https://doi.org/10.1016/j.jhazmat.2012.10.029 DOI: 10.1016/j.jhazmat.2012.10.029]</ref> | |
| − | + | *Sonolysis<ref name="Campbell2015">Campbell, T., Hoffmann, M.R., 2015. Sonochemical degradation of perfluorinated surfactants: Power and multiple frequency effects. Separation and Purification Technology, 156(3), pp. 1019-1027. [https://doi.org/10.1016/j.seppur.2015.09.053 DOI: 10.1016/j.seppur.2015.09.053] Free download from: [https://www.researchgate.net/profile/Tammy_Campbell5/publication/282583363_Sonochemical_Degradation_of_Perfluorinated_Surfactants_Power_and_Multiple_Frequency_Effects/links/5bfc40bd92851cbcdd74449b/Sonochemical-Degradation-of-Perfluorinated-Surfactants-Power-and-Multiple-Frequency-Effects.pdf ResearchGate]</ref><ref name="Cheng2010">Cheng, J., Vecitis, C.D., Park, H., Mader, B.T., Hoffmann, M.R., 2010. Sonochemical Degradation of Perfluorooctane Sulfonate (PFOS) and Perfluorooctanoate (PFOA) in Groundwater: Kinetic Effects of Matrix Inorganics. Environmental Science and Technology, 44(1), pp. 445-450. [https://doi.org/10.1021/es902651g DOI: 10.1021/es902651g]</ref><ref name="Gole2018a">Gole, V.L., Sierra-Alvarez, R., Peng, H., Giesy, J.P., Deymier, P., Keswani, M., 2018. Sono-chemical treatment of per- and poly-fluoroalkyl compounds in aqueous film-forming foams by use of a large-scale multi-transducer dual-frequency based acoustic reactor. Ultrasonics Sonochemistry, 45, pp. 213-222. [https://doi.org/10.1016/j.ultsonch.2018.02.014 DOI: 10.1016/j.ultsonch.2018.02.014] [https://www.sciencedirect.com/science/article/pii/S1350417718301937 Open access article.] [//www.enviro.wiki/images/f/f0/Gole2018a.pdf Report.pdf]</ref><ref name="Gole2018b">Gole, V.L., Fishgold, A., Sierra-Alvarez, R., Deymier, P., Keswani, M., 2018. Treatment of perfluorooctane sulfonic acid (PFOS) using a large-scale sonochemical reactor. Separation and Purification Technology, 194, pp. 104-110. [https://doi.org/10.1016/j.seppur.2017.11.009 DOI: 10.1016/j.seppur.2017.11.009]</ref> | |
| − | * Electro-oxidation<ref name="Zhang2016">Zhang, C., Tang, J., Peng, C., and Jin, M., 2016. Degradation of perfluorinated compounds in wastewater treatment plant effluents by electrochemical oxidation with Nano-ZnO coated electrodes. Journal of Molecular Liquids, 221, pp. 1145-1150. [https://doi.org/10.1016/j.molliq.2016.06.093 DOI: 10.1016/j.molliq.2016.06.093]</ref><ref name="Urtiaga2015">Urtiaga, A., Fernández-González, C., Gómez-Lavín, S., and Ortiz, I., 2015. Kinetics of the electrochemical mineralization of perfluorooctanoic acid on ultrananocrystalline boron doped conductive diamond electrodes. Chemosphere, 129, pp. 20-26. [https://doi.org/10.1016/j.chemosphere.2014.05.090 DOI: 10.1016/j.chemosphere.2014.05.090]</ref><ref name="Schaefer2018">Schaefer, C.E., Choyke, S., Ferguson, P.L., Andaya, C., Burant, A., Maizel, A., Strathmann, T.J. and Higgins, C.P., 2018. Electrochemical Transformations of Perfluoroalkyl Acid (PFAA) Precursors and PFAAs in Groundwater Impacted with Aqueous Film Forming Foams. Environmental Science and Technology, 52(18), pp. 10689-10697. [https://doi.org/10.1021/acs.est.8b02726 DOI: 10.1021/acs.est.8b02726]</ref> | + | *[[Supercritical Water Oxidation (SCWO)]] |
| − | * Heat activated persulfate<ref name="Park2016">Park, S., Lee, L.S., Medina, V. F., Zull, A., and Waisner, S., 2016. Heat-activated persulfate oxidation of PFOA, 6: 2 fluorotelomer sulfonate, and PFOS under conditions suitable for in-situ groundwater remediation. Chemosphere, 145, pp. 376-383. [https://doi.org/10.1016/j.chemosphere.2015.11.097 DOI: 10.1016/j.chemosphere.2015.11.097]</ref> | ||
| − | * Alkaline peroxone<ref name="Lin2012">Lin, A.Y.C., Panchangam, S.C., Chang, C.Y., Hong, P.A., and Hsueh, H.F., 2012. Removal of perfluorooctanoic acid and perfluorooctane sulfonate via ozonation under alkaline condition. Journal of Hazardous Materials, 243, pp. 272-277. [https://doi.org/10.1016/j.jhazmat.2012.10.029 DOI: 10.1016/j.jhazmat.2012.10.029]</ref> | ||
| − | * Sonolysis<ref name="Campbell2015">Campbell, T., Hoffmann, M.R., 2015. Sonochemical degradation of perfluorinated surfactants: Power and multiple frequency effects. Separation and Purification Technology, 156(3), pp. 1019-1027. [https://doi.org/10.1016/j.seppur.2015.09.053 DOI: 10.1016/j.seppur.2015.09.053] Free download from: [https://www.researchgate.net/profile/Tammy_Campbell5/publication/282583363_Sonochemical_Degradation_of_Perfluorinated_Surfactants_Power_and_Multiple_Frequency_Effects/links/5bfc40bd92851cbcdd74449b/Sonochemical-Degradation-of-Perfluorinated-Surfactants-Power-and-Multiple-Frequency-Effects.pdf ResearchGate]</ref><ref name="Cheng2010">Cheng, J., Vecitis, C.D., Park, H., Mader, B.T., Hoffmann, M.R., 2010. Sonochemical Degradation of Perfluorooctane Sulfonate (PFOS) and Perfluorooctanoate (PFOA) in Groundwater: Kinetic Effects of Matrix Inorganics. Environmental Science and Technology, 44(1), pp. 445-450. [https://doi.org/10.1021/es902651g DOI: 10.1021/es902651g]</ref><ref name="Gole2018a">Gole, V.L., Sierra-Alvarez, R., Peng, H., Giesy, J.P., Deymier, P., Keswani, M., 2018. Sono-chemical treatment of per- and poly-fluoroalkyl compounds in aqueous film-forming foams by use of a large-scale multi-transducer dual-frequency based acoustic reactor. Ultrasonics Sonochemistry, 45, pp. 213-222. [https://doi.org/10.1016/j.ultsonch.2018.02.014 DOI: 10.1016/j.ultsonch.2018.02.014] [https://www.sciencedirect.com/science/article/pii/S1350417718301937 Open access article.] [ | ||
| − | * [[Supercritical Water Oxidation (SCWO)]] | ||
|- | |- | ||
| − | | Maturing and< | + | |Maturing and<br>Demonstrated |
| | | | ||
| − | * Chemical coagulation<ref name="Cornelsen2015">Cornelsen Ltd., 2015. PerfluorAd, PFC Water Treatment Solution (product sales site). [http://www.cornelsen.co.uk/perfluorad-pfc-treatment/ Website]</ref> | + | *Chemical coagulation<ref name="Cornelsen2015">Cornelsen Ltd., 2015. PerfluorAd, PFC Water Treatment Solution (product sales site). [http://www.cornelsen.co.uk/perfluorad-pfc-treatment/ Website]</ref> |
| − | * Electrocoagulation<ref name="Wang2016">Wang, Y., Lin, H., Jin, F., Niu, J., Zhao, J., Bi, Y., and Li, Y., 2016. Electrocoagulation mechanism of perfluorooctanoate (PFOA) on a zinc anode: Influence of cathodes and anions. Science of the Total Environment, 557, pp. 542-550. [https://doi.org/10.1016/j.scitotenv.2016.03.114 DOI: 10.1016/j.scitotenv.2016.03.114]</ref> | + | *Electrocoagulation<ref name="Wang2016">Wang, Y., Lin, H., Jin, F., Niu, J., Zhao, J., Bi, Y., and Li, Y., 2016. Electrocoagulation mechanism of perfluorooctanoate (PFOA) on a zinc anode: Influence of cathodes and anions. Science of the Total Environment, 557, pp. 542-550. [https://doi.org/10.1016/j.scitotenv.2016.03.114 DOI: 10.1016/j.scitotenv.2016.03.114]</ref> |
| − | * Foam fractionation<ref name="Horst2018">Horst, J., McDonough, J., Ross, I., Dickson, M., Miles, J., Hurst, J., and Storch, P., 2018. Water Treatment Technologies for PFAS: The Next Generation. Groundwater Monitoring and Remediation, 38(2), pp. 13-23. [https://doi.org/10.1111/gwmr.12281 DOI: 10.1111/gwmr.12281]</ref><ref name="EPC2017">EPC Media Group Pty Ltd., 2017. OPEC systems delivers PFAS contamination breakthrough. Waste + Water Management Australia, 44(3), 26-27. [https://search.informit.org/doi/10.3316/informit.253699294687114 DOI: 10.3316/informit.253699294687114] ISSN: 1838-7098</ref> | + | *Foam fractionation<ref name="Horst2018">Horst, J., McDonough, J., Ross, I., Dickson, M., Miles, J., Hurst, J., and Storch, P., 2018. Water Treatment Technologies for PFAS: The Next Generation. Groundwater Monitoring and Remediation, 38(2), pp. 13-23. [https://doi.org/10.1111/gwmr.12281 DOI: 10.1111/gwmr.12281]</ref><ref name="EPC2017">EPC Media Group Pty Ltd., 2017. OPEC systems delivers PFAS contamination breakthrough. Waste + Water Management Australia, 44(3), 26-27. [https://search.informit.org/doi/10.3316/informit.253699294687114 DOI: 10.3316/informit.253699294687114] ISSN: 1838-7098</ref> |
| | | | ||
| − | * Low temperature plasma<ref name="Stratton2017">Stratton, G.R., Dai, F., Bellona, C.L., Holsen, T.M., Dickenson, E.R., and Mededovic Thagard, S., 2017. Plasma-Based Water Treatment: Efficient Transformation of Perfluoroalkyl Substances in Prepared Solutions and Contaminated Groundwater. Environmental Science and Technology, 51(3), pp. 1643-1648. [https://doi.org/10.1021/acs.est.6b04215 DOI: 10.1021/acs.est.6b04215]</ref><ref name="Singh2019">Singh, R.K., Multari, N., Nau-Hix, C., Anderson, R.H., Richardson, S.D., Holsen, T.M. and Mededovic Thagard, S., 2019. Rapid Removal of Poly- and Perfluorinated Compounds from Investigation-Derived Waste (IDW) in a Pilot-Scale Plasma Reactor. Environmental Science and Technology, 53(19), pp. 11375-11382. [https://doi.org/10.1021/acs.est.9b02964 DOI: 10.1021/acs.est.9b02964]</ref> | + | *Low temperature plasma<ref name="Stratton2017">Stratton, G.R., Dai, F., Bellona, C.L., Holsen, T.M., Dickenson, E.R., and Mededovic Thagard, S., 2017. Plasma-Based Water Treatment: Efficient Transformation of Perfluoroalkyl Substances in Prepared Solutions and Contaminated Groundwater. Environmental Science and Technology, 51(3), pp. 1643-1648. [https://doi.org/10.1021/acs.est.6b04215 DOI: 10.1021/acs.est.6b04215]</ref><ref name="Singh2019">Singh, R.K., Multari, N., Nau-Hix, C., Anderson, R.H., Richardson, S.D., Holsen, T.M. and Mededovic Thagard, S., 2019. Rapid Removal of Poly- and Perfluorinated Compounds from Investigation-Derived Waste (IDW) in a Pilot-Scale Plasma Reactor. Environmental Science and Technology, 53(19), pp. 11375-11382. [https://doi.org/10.1021/acs.est.9b02964 DOI: 10.1021/acs.est.9b02964]</ref> |
|- | |- | ||
| − | | colspan="3" style="background:white;" | * There are several other destructive technologies such as alternative oxidants, and activation< | + | | colspan="3" style="background:white;" |* There are several other destructive technologies such as alternative oxidants, and activation<br>methods of oxidants, but for the purpose of this article, the main categories are presented here. |
|} | |} | ||
Numerous separation and destructive technologies are in the developmental stages of bench-scale testing or limited field-scale demonstrations. Some of these are listed in Table 1 and defined below. | Numerous separation and destructive technologies are in the developmental stages of bench-scale testing or limited field-scale demonstrations. Some of these are listed in Table 1 and defined below. | ||
| − | *[[Wikipedia: Biochar |'''Biochar''']] is charcoal produced from cellulosic biomass by pyrolysis in an oxygen-free environment. Without additional chemical or physical treatment, biochar is not quite as effective at adsorbing some contaminants as granular activated carbon (GAC)<ref name="Guo2017"/><ref name="Kupryianchyk2016"/><ref name="Inyang2017"/>. However, GAC is typically produced from high quality hardwood charcoal or coal while biochar can be made from locally available feedstocks or waste streams such as paper mill waste or agricultural waste. Biochar has a long history of agricultural use as a soil amendment. | + | *[[Wikipedia: Biochar |'''Biochar''']] is charcoal produced from cellulosic biomass by pyrolysis in an oxygen-free environment. Without additional chemical or physical treatment, biochar is not quite as effective at adsorbing some contaminants as granular activated carbon (GAC)<ref name="Guo2017" /><ref name="Kupryianchyk2016" /><ref name="Inyang2017" />. However, GAC is typically produced from high quality hardwood charcoal or coal while biochar can be made from locally available feedstocks or waste streams such as paper mill waste or agricultural waste. Biochar has a long history of agricultural use as a soil amendment. |
| − | *[[Wikipedia: Zeolite |'''Zeolites''']] are microporous, aluminosilicate minerals such as thomsonite and stilbite which can remove contaminants through adsorbent, molecular sieve, and/or ion exchange interactions. Some types of zeolites occur naturally but many more are synthetically produced for a wide variety of industrial uses. | + | *[[Wikipedia: Zeolite |'''Zeolites''']] are microporous, aluminosilicate minerals such as thomsonite and stilbite which can remove contaminants through adsorbent, molecular sieve, and/or ion exchange interactions. Some types of zeolites occur naturally but many more are synthetically produced for a wide variety of industrial uses. |
| − | *'''Specialty adsorbents''' such as cross-linked [[Wikipedia: Chitosan |chitosan]] beads may offer improved adsorption capacity or kinetics<ref name="Zhang2011"/>, while bench-scale studies suggest TiO<sub>2</sub> nanotubes can adsorb PFAS and subsequently serve as a photocatalyst for their destruction<ref name="Hu2016"/>. | + | *'''Specialty adsorbents''' such as cross-linked [[Wikipedia: Chitosan |chitosan]] beads may offer improved adsorption capacity or kinetics<ref name="Zhang2011" />, while bench-scale studies suggest TiO<sub>2</sub> nanotubes can adsorb PFAS and subsequently serve as a photocatalyst for their destruction<ref name="Hu2016" />. |
| − | *'''Chemical coagulation''' refers to the addition of a chemical such as [[Wikipedia: Alum |alum]] or [[Wikipedia: Iron(III) chloride |ferric chloride]] to neutralize the unbalanced charges that would otherwise help keep heavy metal ions and colloidal solids in suspension through electrostatic repulsion. Once the electrostatic force is neutralized, particles begin to aggregate into flocs and settle out of suspension. Bench-scale studies suggest that 25% to 30% of the polar molecules perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) could be removed by using approximately twice the usual dose of traditional coagulants<ref name="Xiao2013">Xiao, F., Simcik, M.F., Gulliver, J.S., 2013. Mechanisms for removal of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) from drinking water by conventional and enhanced coagulation. Water Research, 47(1), pp. 49-56. [https://doi.org/10.1016/j.watres.2012.09.024 | + | *'''Chemical coagulation''' refers to the addition of a chemical such as [[Wikipedia: Alum |alum]] or [[Wikipedia: Iron(III) chloride |ferric chloride]] to neutralize the unbalanced charges that would otherwise help keep heavy metal ions and colloidal solids in suspension through electrostatic repulsion. Once the electrostatic force is neutralized, particles begin to aggregate into flocs and settle out of suspension. Bench-scale studies suggest that 25% to 30% of the polar molecules perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) could be removed by using approximately twice the usual dose of traditional coagulants<ref name="Xiao2013">Xiao, F., Simcik, M.F., Gulliver, J.S., 2013. Mechanisms for removal of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) from drinking water by conventional and enhanced coagulation. Water Research, 47(1), pp. 49-56. [https://doi.org/10.1016/j.watres.2012.09.024 DOI: 10.1016/j.watres.2012.09.024] Free download available from [https://www.researchgate.net/profile/Md_Washim_Akram/post/Any-work-done-of-removal-of-low-levels-of-PFOS-from-drinking-water-using-RO-membranes/attachment/5cb372273843b01b9b99f950/AS%3A747645723242497%401555264039487/download/xiao2013.pdf ResearchGate]</ref>. Initial treatment of higher concentrations of polar PFAS by coagulation may prove useful to reduce loading on subsequent removal processes such as activated carbon adsorption or anion exchange which can achieve much lower effluent PFAS concentrations. |
| − | *'''Electrocoagulation''' neutralizes electrostatic repulsion using DC power rather than chemical coagulants. As current passes between submerged electrodes, metal ions are released from the sacrificial anode that counter the unbalanced charges of very small particles in suspension which can then coagulate into flocs. Some contaminants are captured in the flocs, while others may be removed by ionization, hydrolysis, or attack by free radicals as they move through the applied electric field<ref name="Wang2016"/>. | + | *'''Electrocoagulation''' neutralizes electrostatic repulsion using DC power rather than chemical coagulants. As current passes between submerged electrodes, metal ions are released from the sacrificial anode that counter the unbalanced charges of very small particles in suspension which can then coagulate into flocs. Some contaminants are captured in the flocs, while others may be removed by ionization, hydrolysis, or attack by free radicals as they move through the applied electric field<ref name="Wang2016" />. |
| − | *'''Foam fractionation''' is used to separate surface active agents (i.e. [[Wikipedia: Surfactant |surfactants]]) and hydrophobic particles from water. Many PFAS have been valued commercially and industrially because of their surfactant or hydrophobic properties. In this technique, small air bubbles are introduced at the bottom of a narrow column of contaminated water. As the bubbles rise through the column, surfactants and hydrophobic materials partition to the air/water interface of the bubbles. As these materials accumulate at the interfaces, a PFAS-rich foam develops which accumulates at the top of the column where it can be easily removed and further processed or disposed<ref name="Horst2018"/><ref name="EPC2017"/>. | + | *'''Foam fractionation''' is used to separate surface active agents (i.e. [[Wikipedia: Surfactant |surfactants]]) and hydrophobic particles from water. Many PFAS have been valued commercially and industrially because of their surfactant or hydrophobic properties. In this technique, small air bubbles are introduced at the bottom of a narrow column of contaminated water. As the bubbles rise through the column, surfactants and hydrophobic materials partition to the air/water interface of the bubbles. As these materials accumulate at the interfaces, a PFAS-rich foam develops which accumulates at the top of the column where it can be easily removed and further processed or disposed<ref name="Horst2018" /><ref name="EPC2017" />. |
| − | *'''Electro-oxidation''' relies on submerged electrodes but uses greater current densities than electrocoagulation with the goal of producing highly reactive [[Wikipedia: Hydroxyl radical |hydroxyl radicals]] (*OH) at the anode surface to destroy PFAS and many other contaminants. A wide variety of anode materials have been studied at the bench scale, each with different PFAS removal efficiencies and durabilities<ref name="Zhang2016"/><ref name="Urtiaga2015"/><ref name="Schaefer2018"/>. Very low salinity waters may require addition of salts to have sufficient ion concentrations to support the electrolytic reaction. | + | *'''Electro-oxidation''' relies on submerged electrodes but uses greater current densities than electrocoagulation with the goal of producing highly reactive [[Wikipedia: Hydroxyl radical |hydroxyl radicals]] (*OH) at the anode surface to destroy PFAS and many other contaminants. A wide variety of anode materials have been studied at the bench scale, each with different PFAS removal efficiencies and durabilities<ref name="Zhang2016" /><ref name="Urtiaga2015" /><ref name="Schaefer2018" />. Very low salinity waters may require addition of salts to have sufficient ion concentrations to support the electrolytic reaction. |
| − | *'''Heat activated [[Wikipedia: Persulfate |persulfate]]''' has a long history of use to oxidize a variety of organic contaminants in soil. A bench scale feasibility study simulating ''in situ'' treatment of PFAS impacted groundwater found that perfluorooctanoic acid (PFOA) was oxidized within 72 hours at 50°C and that the rate of oxidation increased with temperature. However, perfluorooctanesulfonic acid (PFOS) was reportedly unaffected by this treatment<ref name="Park2016"/>. | + | *'''Heat activated [[Wikipedia: Persulfate |persulfate]]''' has a long history of use to oxidize a variety of organic contaminants in soil. A bench scale feasibility study simulating ''in situ'' treatment of PFAS impacted groundwater found that perfluorooctanoic acid (PFOA) was oxidized within 72 hours at 50°C and that the rate of oxidation increased with temperature. However, perfluorooctanesulfonic acid (PFOS) was reportedly unaffected by this treatment<ref name="Park2016" />. |
| − | *'''Alkaline peroxone''' treatment has been used to remove PFOA and PFOS from electronics fabrication industry wastewater in Taiwan. Degradation rates of 85% to 100% were reported using peroxone, a combination of ozone (O<sub>3</sub>) and peroxide (H<sub>2</sub>O<sub>2</sub>), with pH elevated to 11. Best results were obtained by ozonating for 15 minutes followed by pH adjustment and 4 more hours of ozonation<ref name="Lin2012"/>. | + | *'''Alkaline peroxone''' treatment has been used to remove PFOA and PFOS from electronics fabrication industry wastewater in Taiwan. Degradation rates of 85% to 100% were reported using peroxone, a combination of ozone (O<sub>3</sub>) and peroxide (H<sub>2</sub>O<sub>2</sub>), with pH elevated to 11. Best results were obtained by ozonating for 15 minutes followed by pH adjustment and 4 more hours of ozonation<ref name="Lin2012" />. |
| − | *'''Sonolysis''' of PFAS is believed to occur primarily at the vapor/water interface of cavitation bubbles caused by application of sonic energy to the contaminated water. Higher degradation rates have been reported for the more hydrophobic PFAS which are more likely to partition to the bubble interface. Degradation rates also increase with applied sonic power density. The presence of other organic or inorganic constituents in the water being treated may drastically reduce the rate of PFOS and PFOA destruction. Sonolysis of PFAS may be most effective at pH of 3 to 4 and when using two frequencies of sonic input simultaneously in a single reactor<ref name="Campbell2015"/><ref name="Cheng2010"/><ref name="Gole2018a"/>. | + | *'''Sonolysis''' of PFAS is believed to occur primarily at the vapor/water interface of cavitation bubbles caused by application of sonic energy to the contaminated water. Higher degradation rates have been reported for the more hydrophobic PFAS which are more likely to partition to the bubble interface. Degradation rates also increase with applied sonic power density. The presence of other organic or inorganic constituents in the water being treated may drastically reduce the rate of PFOS and PFOA destruction. Sonolysis of PFAS may be most effective at pH of 3 to 4 and when using two frequencies of sonic input simultaneously in a single reactor<ref name="Campbell2015" /><ref name="Cheng2010" /><ref name="Gole2018a" />. |
*'''[[Supercritical Water Oxidation (SCWO)]]''' takes advantage of the unique properties of water in the supercritical phase (temperature ≥ 374°C and pressure ≥ 218 atm) which acts like a dense non-polar solvent with the transport qualities of a gas. Because oxygen is fully miscible in supercritical water, organic contaminants can be fully oxidized quickly. The oxidation of organics is an exothermic reaction, and the released heat energy can be harnessed to make a SCWO system self-sustaining after startup, if the influent waste stream is sufficiently concentrated. | *'''[[Supercritical Water Oxidation (SCWO)]]''' takes advantage of the unique properties of water in the supercritical phase (temperature ≥ 374°C and pressure ≥ 218 atm) which acts like a dense non-polar solvent with the transport qualities of a gas. Because oxygen is fully miscible in supercritical water, organic contaminants can be fully oxidized quickly. The oxidation of organics is an exothermic reaction, and the released heat energy can be harnessed to make a SCWO system self-sustaining after startup, if the influent waste stream is sufficiently concentrated. | ||
| − | *When a normally neutral and non-conductive fluid is heated sufficiently or subjected to a strong enough electromagnetic field, some electrons are stripped from their nucleus creating a highly charged and electrically conductive gas of ions and free electrons known as a [[Wikipedia: Plasma (physics) |plasma]]. '''Low temperature plasma''' treatment relies on a continuous electric discharge (i.e. spark) to create a localized plasma that contaminated water can be circulated through to destroy many organic compounds. One bench scale study reported removing 90% of PFOA after 30 minutes of plasma treatment<ref name="Stratton2017"/>. In another study, a pilot scale plasma reactor treating moderately to highly PFAS-impacted water samples reportedly reduced PFOS and PFOA concentrations to below the US EPA’s health advisory limits (HALs) in two thirds of the samples with less than one minute of treatment. The most contaminated samples and the most conductive samples required up to 50 minutes of treatment<ref name="Singh2019"/>. | + | *When a normally neutral and non-conductive fluid is heated sufficiently or subjected to a strong enough electromagnetic field, some electrons are stripped from their nucleus creating a highly charged and electrically conductive gas of ions and free electrons known as a [[Wikipedia: Plasma (physics) |plasma]]. '''Low temperature plasma''' treatment relies on a continuous electric discharge (i.e. spark) to create a localized plasma that contaminated water can be circulated through to destroy many organic compounds. One bench scale study reported removing 90% of PFOA after 30 minutes of plasma treatment<ref name="Stratton2017" />. In another study, a pilot scale plasma reactor treating moderately to highly PFAS-impacted water samples reportedly reduced PFOS and PFOA concentrations to below the US EPA’s health advisory limits (HALs) in two thirds of the samples with less than one minute of treatment. The most contaminated samples and the most conductive samples required up to 50 minutes of treatment<ref name="Singh2019" />. |
==Conclusions== | ==Conclusions== | ||
Latest revision as of 10:34, 30 October 2024
Well-developed ex situ treatment technologies applicable to treatment of perfluoroalkyl and polyfluoroalkyl substances (PFAS) in drinking water and non-potable groundwater include membrane filtration (reverse osmosis or RO and nanofiltration or NF), activated carbon adsorption (granular and powdered), and anion exchange. However, these technologies are less demonstrated for removal of PFAS from more complex matrices such as wastewater and leachate. There are also a variety of separation and destructive technologies in various stages of development. Some of these processes may also be applicable to more complex matrices including wastewater and landfill leachate.
Related Article(s):
- Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS)
- PFAS Soil Remediation Technologies
- PFAS Sources
- PFAS Transport and Fate
- PFAS Treatment by Anion Exchange
- PFAS Treatment by Electrical Discharge Plasma
- Photoactivated Reductive Defluorination - PFAS Destruction
- Supercritical Water Oxidation (SCWO)
Contributor(s): Dr. Scott Grieco and James Hatton
Key Resource(s):
Established PFAS Treatment Technologies
Three technologies are well demonstrated for removal of PFAS from drinking water and non-potable groundwater (as described below):
- membrane filtration including reverse osmosis (RO) and nanofiltration (NF)
- granular activated carbon (GAC) and powdered activated carbon (PAC) adsorption
- anion exchange (IX)
However, these technologies are less demonstrated for removal of PFAS from more complex matrices such as wastewater and leachate. Site-specific considerations that affect the selection of optimum treatment technologies for a given site include water chemistry, required flow rate, treatment criteria, waste residual generation, residual disposal options, and operational complexity. Treatability studies with site water are highly recommended because every site has different factors that may affect engineering design for these technologies.
Membrane Filtration
Given their ability to remove dissolved contaminants at a molecular size level, RO and some NF membranes can be highly effective for PFAS removal. For RO systems (Figure 1), several studies have demonstrated effective removal of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) (see PFAS for nomenclature) from drinking water with removal rates well above 90%[5][6][7]. RO potable water reuse treatment systems implemented in California have also demonstrated effective PFOS and PFOA removal as reported by the Water Research Foundation (WRF)[1]. Analysis of permeate at both sites referenced by the WRF confirmed that short and long chain PFAS concentrations in the treated water were reduced to levels below test method reporting limits.
Full-scale studies using larger effective pore size NF membranes for PFAS removal are limited in number but are promising since NF systems are somewhat less costly than RO and may be nearly as effective in removing PFAS. Recent laboratory or pilot studies have shown good performance of NF membranes[8][9][10][11][12].
Although membrane RO and NF processes are generally capable of providing uniform removal rates relative to short and long chain PFAS compounds (see PFAS for nomenclature), other aspects of these treatment technologies are more challenging:
- Membranes must be flushed and cleaned periodically, such that overall water recovery rates (process water volumes consumed, wasted, and lost vs. treated water volumes produced) are much lower than those for GAC and IX processes. Membrane fouling can be slowed or avoided depending on operating conditions, membrane modifications, and feed modifications[13]. Typically, 70-90% of the water supplied into a membrane RO process is recoverable as treated water. The remaining 10-30% is reject containing approximately 4 to 8 times the initial PFAS concentration (depending on recovery rate).
- These cleaning and flushing processes create a continuous liquid waste stream, which periodically includes harsh membrane cleaning chemicals as well as a continuous flow of concentrated membrane reject chemicals (i.e., PFAS) that must be properly managed and disposed of. Management often includes further treatment to remove PFAS from the liquid waste.
- RO and NF systems are inherently more expensive and complicated systems to implement, operate, and maintain compared to adsorption processes. Treatment system operator certification and process monitoring requirements are correspondingly markedly higher for RO and NF than they are for GAC and IX.
- Water feed pressures required to drive flow through membrane RO and NF processes are considerably higher than those involved with GAC and IX processes. This results in reduced process efficiency and higher pumping and electrical operating costs.
- Membrane systems can also be subject to issues with irreversible membrane fouling, clogging, and scaling or other physical membrane damage and failures. Additional water pretreatment and higher levels of monitoring and maintenance are then required, further adding to the higher costs of such systems.
Activated Carbon Adsorption
Activated carbon is a form of carbon processed to have small pores that increase the surface area available for adsorption of constituents from water. Activated carbon is derived from many source materials, including coconut shells, wood, lignite, and bituminous coal. Different types of activated carbon base materials have varied adsorption characteristics such that some may be better suited to removing certain contaminant compounds than others. Results from laboratory testing, pilot evaluations, and full-scale system operations suggest that bituminous coal-based GAC is generally the best performing carbon for PFAS removal[14][15].
The removal efficiency of individual PFAS compounds using GAC is a function of both the PFAS functional group (carboxylic acid versus sulfonic acid) and also the perfluoro-carbon chain length[16][17](see PFAS for nomenclature):
- perfluoro-sulfonate acids (PFSAs) are more efficiently removed than perfluoro-carboxylic acids (PFCAs) of the same chain length
- long chain compounds of the same functional group are removed better than the shorter chains
Activated carbon may be applied in drinking water systems as GAC or PAC[18][19]. GAC has larger granules and is reusable, while PAC has much smaller granules and is not typically reused. PAC has most often been used as a temporary treatment because costs associated with disposal and replacement of the used PAC tend to preclude using it for long-term treatment. A typical GAC installation for a private drinking water well is shown in Figure 2. Contrary to PAC, GAC used to treat PFAS can be reactivated by the manufacturer, driving the PFAS from the GAC and into off-gas. The extracted gas is then treated with thermal oxidation (temperatures often 1200°C to 1400°C). The reactivated GAC is then brought back to the site and reused. Thus, GAC can ultimately be a destructive treatment technology.
Anion Exchange
Anion exchange has also been demonstrated for the adsorption of PFAS, and published results note higher sorption per pound than GAC[16][20][21]. The higher capacity is believed to be due to combined hydrophobic and ion exchange adsorption mechanisms, whereas GAC mainly relies on hydrophobic attraction. Anion exchange resins can be highly selective, or they can also remove other contaminants based on design requirements and water chemistry. Resins have greater affinity for PFAS subgroup PFSA than for PFCA, and affinity increases with carbon chain length. Anion exchange resins are a viable alternative to GAC for ex situ treatment of PFAS anions, and several venders sell resins capable of removing PFAS. Resins available for treating PFAS include regenerable resins that can be used multiple times (Figure 3) and single-use resins that must be disposed or destroyed after use[20]. Regenerable resins generate a solvent and brine solution, which is distilled to recover the solvent prior to the brine being adsorbed onto a small quantity of GAC or resin for ultimate disposal. This use of one treatment technology (GAC, IX) to support another (RO) is sometimes referred to as a “treatment train” approach. Single-use resins can be more fully exhausted than regenerable resins can and may be a more cost-effective solution for low concentration PFAS contamination, while regenerable resins may be more cost effective for higher concentration contamination.
Developing PFAS Treatment Technologies
| Stage | Separation/Transfer | Destructive* |
|---|---|---|
| Developing | ||
| Maturing and Demonstrated |
||
| * There are several other destructive technologies such as alternative oxidants, and activation methods of oxidants, but for the purpose of this article, the main categories are presented here. | ||
Numerous separation and destructive technologies are in the developmental stages of bench-scale testing or limited field-scale demonstrations. Some of these are listed in Table 1 and defined below.
- Biochar is charcoal produced from cellulosic biomass by pyrolysis in an oxygen-free environment. Without additional chemical or physical treatment, biochar is not quite as effective at adsorbing some contaminants as granular activated carbon (GAC)[22][23][24]. However, GAC is typically produced from high quality hardwood charcoal or coal while biochar can be made from locally available feedstocks or waste streams such as paper mill waste or agricultural waste. Biochar has a long history of agricultural use as a soil amendment.
- Zeolites are microporous, aluminosilicate minerals such as thomsonite and stilbite which can remove contaminants through adsorbent, molecular sieve, and/or ion exchange interactions. Some types of zeolites occur naturally but many more are synthetically produced for a wide variety of industrial uses.
- Specialty adsorbents such as cross-linked chitosan beads may offer improved adsorption capacity or kinetics[27], while bench-scale studies suggest TiO2 nanotubes can adsorb PFAS and subsequently serve as a photocatalyst for their destruction[29].
- Chemical coagulation refers to the addition of a chemical such as alum or ferric chloride to neutralize the unbalanced charges that would otherwise help keep heavy metal ions and colloidal solids in suspension through electrostatic repulsion. Once the electrostatic force is neutralized, particles begin to aggregate into flocs and settle out of suspension. Bench-scale studies suggest that 25% to 30% of the polar molecules perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) could be removed by using approximately twice the usual dose of traditional coagulants[45]. Initial treatment of higher concentrations of polar PFAS by coagulation may prove useful to reduce loading on subsequent removal processes such as activated carbon adsorption or anion exchange which can achieve much lower effluent PFAS concentrations.
- Electrocoagulation neutralizes electrostatic repulsion using DC power rather than chemical coagulants. As current passes between submerged electrodes, metal ions are released from the sacrificial anode that counter the unbalanced charges of very small particles in suspension which can then coagulate into flocs. Some contaminants are captured in the flocs, while others may be removed by ionization, hydrolysis, or attack by free radicals as they move through the applied electric field[40].
- Foam fractionation is used to separate surface active agents (i.e. surfactants) and hydrophobic particles from water. Many PFAS have been valued commercially and industrially because of their surfactant or hydrophobic properties. In this technique, small air bubbles are introduced at the bottom of a narrow column of contaminated water. As the bubbles rise through the column, surfactants and hydrophobic materials partition to the air/water interface of the bubbles. As these materials accumulate at the interfaces, a PFAS-rich foam develops which accumulates at the top of the column where it can be easily removed and further processed or disposed[41][42].
- Electro-oxidation relies on submerged electrodes but uses greater current densities than electrocoagulation with the goal of producing highly reactive hydroxyl radicals (*OH) at the anode surface to destroy PFAS and many other contaminants. A wide variety of anode materials have been studied at the bench scale, each with different PFAS removal efficiencies and durabilities[30][31][32]. Very low salinity waters may require addition of salts to have sufficient ion concentrations to support the electrolytic reaction.
- Heat activated persulfate has a long history of use to oxidize a variety of organic contaminants in soil. A bench scale feasibility study simulating in situ treatment of PFAS impacted groundwater found that perfluorooctanoic acid (PFOA) was oxidized within 72 hours at 50°C and that the rate of oxidation increased with temperature. However, perfluorooctanesulfonic acid (PFOS) was reportedly unaffected by this treatment[33].
- Alkaline peroxone treatment has been used to remove PFOA and PFOS from electronics fabrication industry wastewater in Taiwan. Degradation rates of 85% to 100% were reported using peroxone, a combination of ozone (O3) and peroxide (H2O2), with pH elevated to 11. Best results were obtained by ozonating for 15 minutes followed by pH adjustment and 4 more hours of ozonation[34].
- Sonolysis of PFAS is believed to occur primarily at the vapor/water interface of cavitation bubbles caused by application of sonic energy to the contaminated water. Higher degradation rates have been reported for the more hydrophobic PFAS which are more likely to partition to the bubble interface. Degradation rates also increase with applied sonic power density. The presence of other organic or inorganic constituents in the water being treated may drastically reduce the rate of PFOS and PFOA destruction. Sonolysis of PFAS may be most effective at pH of 3 to 4 and when using two frequencies of sonic input simultaneously in a single reactor[35][36][37].
- Supercritical Water Oxidation (SCWO) takes advantage of the unique properties of water in the supercritical phase (temperature ≥ 374°C and pressure ≥ 218 atm) which acts like a dense non-polar solvent with the transport qualities of a gas. Because oxygen is fully miscible in supercritical water, organic contaminants can be fully oxidized quickly. The oxidation of organics is an exothermic reaction, and the released heat energy can be harnessed to make a SCWO system self-sustaining after startup, if the influent waste stream is sufficiently concentrated.
- When a normally neutral and non-conductive fluid is heated sufficiently or subjected to a strong enough electromagnetic field, some electrons are stripped from their nucleus creating a highly charged and electrically conductive gas of ions and free electrons known as a plasma. Low temperature plasma treatment relies on a continuous electric discharge (i.e. spark) to create a localized plasma that contaminated water can be circulated through to destroy many organic compounds. One bench scale study reported removing 90% of PFOA after 30 minutes of plasma treatment[43]. In another study, a pilot scale plasma reactor treating moderately to highly PFAS-impacted water samples reportedly reduced PFOS and PFOA concentrations to below the US EPA’s health advisory limits (HALs) in two thirds of the samples with less than one minute of treatment. The most contaminated samples and the most conductive samples required up to 50 minutes of treatment[44].
Conclusions
The well established processes for removing PFAS from water all produce residuals that require management, and it is likely that newer processes under development will also produce some residuals. Often, it is the residuals that limit the usefulness of the process. For instance, RO and NF may currently provide the most complete treatment of water, but the production of a relatively high volume of PFAS-containing liquid reject (the portion of the liquid that retains the contaminants and is “rejected” from the process) limits their application. Often, a second treatment technology such as an adsorbent is required to support the main technology by concentrating or treating the residuals.
As more testing and operational data on adsorbents are generated, it is becoming evident that no adsorbent technology outperforms the others in all cases. Whether GAC, ion exchange or another technology is the most technically efficient and cost effective long term option for a given site depends on influent water geochemistry and contaminant concentrations, treatment standards, co-contaminants, duration of treatment, and required flow rates. New generation adsorbents are rapidly being introduced into the market at “evaluation scale” which may provide advantages over commercially available adsorbents.
Several newer technologies are being evaluated in the lab and in the field which include electro-oxidation, heat-activated persulfate, sonolysis, electrocoagulation, low temperature plasma, supercritical water oxidation, and foam fractionation. These and other potential treatments for PFAS are still largely in the developmental stage. Several technologies show promise for improved management of PFAS sites. However, it is unlikely that a single technology will be adequate for full remediation at many sites. A multi-technology treatment train approach may be necessary for effective treatment of this complicated group of compounds.
References
- ^ 1.0 1.1 Dickenson, E. and Higgins, C., 2016. Treatment Mitigation Strategies for Poly- and Perfluoroalkyl Substances, Report Number 4322. Water Research Foundation, Denver, Colorado. 123 pages. ISBN 978-1-60573-234-3
- ^ Interstate Technology and Regulatory Council (ITRC), 2020. PFAS Technical and Regulatory Guidance Document and Fact Sheets, PFAS-1. PFAS Team, Washington, DC. Website Report.pdf
- ^ Kucharzyk, K.H., Darlington, R., Benotti, M., Deeb, R. and Hawley, E., 2017. Novel treatment technologies for PFAS compounds: A critical review. Journal of Environmental Management, 204(2), pp. 757-764. DOI: 10.1016/j.jenvman.2017.08.016 Manuscript available from: ResearchGate.
- ^ Merino, N., Qu, Y., Deeb, R.A., Hawley, E.L., Hoffmann, M.R., and Mahendra, S., 2016. Degradation and Removal Methods for Perfluoroalkyl and Polyfluoroalkyl Substances in Water. Environmental Engineering Science, 33(9), pp. 615-649. DOI: 10.1089/ees.2016.0233
- ^ Tang, C.Y., Fu, Q.S., Robertson, A.P., Criddle, C.S., and Leckie, J.O., 2006. Use of Reverse Osmosis Membranes to Remove Perfluorooctane Sulfonate (PFOS) from Semiconductor Wastewater. Environmental Science and Technology, 40(23), pp. 7343-7349. DOI: 10.1021/es060831q
- ^ Flores, C., Ventura, F., Martin-Alonso, J., and Caixach, J., 2013. Occurrence of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in NE Spanish surface waters and their removal in a drinking water treatment plant that combines conventional and advanced treatments in parallel lines. Science of the Total environment, 461, 618-626. DOI: 10.1016/j.scitotenv.2013.05.026
- ^ Appleman, T.D., Higgins, C.P., Quiñones, O., Vanderford, B.J., Kolstad, C., Zeigler-Holady, J.C., and Dickenson, E.R., 2014. Treatment of poly- and perfluoroalkyl substances in US full-scale water treatment systems. Water Research, 51, pp. 246-255. DOI: 10.1016/j.watres.2013.10.067
- ^ Steinle-Darling, E., and Reinhard, M., 2008. Nanofiltration for Trace Organic Contaminant Removal: Structure, Solution, and Membrane Fouling Effects on the Rejection of Perfluorochemicals. Environmental Science and Technology, 42(14), pp. 5292-5297. DOI: 10.1021/es703207s Free download from: Academia.
- ^ Appleman, T.D., Dickenson, E.R., Bellona, C., and Higgins, C.P., 2013. Nanofiltration and granular activated carbon treatment of perfluoroalkyl acids. Journal of Hazardous Materials, 260, 740-746. DOI: 10.1016/j.jhazmat.2013.06.033
- ^ Soriano, Á., Gorri, D., and Urtiaga, A., 2017. Efficient treatment of perfluorohexanoic acid by nanofiltration followed by electrochemical degradation of the NF concentrate. Water Research, 112, 147-156. DOI: 10.1016/j.watres.2017.01.043 Author’s Manuscript.
- ^ Zeng, C., Tanaka, S., Suzuki, Y., Yukioka, S., and Fujii, S., 2017. Rejection of Trace Level Perfluorohexanoic Acid (PFHxA) in Pure Water by Loose Nanofiltration Membrane. Journal of Water and Environment Technology, 15(3), pp. 120-127. DOI: 10.2965/jwet.16-072 Free download from: J-STAGE
- ^ Wang, J., Wang, L., Xu, C., Zhi, R., Miao, R., Liang, T., Yue, X., Lv, Y. and Liu, T., 2018. Perfluorooctane sulfonate and perfluorobutane sulfonate removal from water by nanofiltration membrane: The roles of solute concentration, ionic strength, and macromolecular organic foulants. Chemical Engineering Journal, 332, pp. 787-797. DOI: 10.1016/j.cej.2017.09.061
- ^ Le Roux, I., Krieg, H.M., Yeates, C.A. and Breytenbach, J.C., 2005. Use of chitosan as an antifouling agent in a membrane bioreactor. Journal of Membrane Science, 248(1-2), pp. 127-136. DOI: 10.1016/j.memsci.2004.10.005
- ^ McNamara, J.D., Franco, R., Mimna, R., and Zappa, L., 2018. Comparison of Activated Carbons for Removal of Perfluorinated Compounds from Drinking Water. Journal‐American Water Works Association, 110(1), pp. E2-E14. DOI: 10.5942/jawwa.2018.110.0003
- ^ Westreich, P., Mimna, R., Brewer, J., and Forrester, F., 2018. The removal of short‐chain and long‐chain perfluoroalkyl acids and sulfonates via granular activated carbons: A comparative column study. Remediation Journal, 29(1), pp. 19-26. DOI: 10.1002/rem.21579
- ^ 16.0 16.1 McCleaf, P., Englund, S., Östlund, A., Lindegren, K., Wiberg, K., and Ahrens, L., 2017. Removal efficiency of multiple poly-and perfluoroalkyl substances (PFASs) in drinking water using granular activated carbon (GAC) and anion exchange (AE) column tests. Water Research, 120, pp. 77-87. DOI: 10.1016/j.watres.2017.04.057
- ^ Eschauzier, C., Beerendonk, E., Scholte-Veenendaal, P., and De Voogt, P., 2012. Impact of Treatment Processes on the Removal of Perfluoroalkyl Acids from the Drinking Water Production Chain. Environmental Science and Technology, 46(3), pp. 1708-1715. DOI: 10.1021/es201662b
- ^ Dudley, L.A., Arevalo, E.C., and Knappe, D.R., 2015. Removal of Perfluoroalkyl Substances by PAC Adsorption and Anion Exchange. Water Research Foundation Project #4344. Free download of Executive Summary from: Water Research Foundation (Public Plus account)
- ^ Qian, J., Shen, M., Wang, P., Wang, C., Li, K., Liu, J., Lu, B. and Tian, X., 2017. Perfluorooctane sulfonate adsorption on powder activated carbon: Effect of phosphate (P) competition, pH, and temperature. Chemosphere, 182, pp. 215-222. DOI: 10.1016/j.chemosphere.2017.05.033
- ^ 20.0 20.1 Senevirathna, S.T.M.L.D., Tanaka, S., Fujii, S., Kunacheva, C., Harada, H., Shivakoti, B.R., and Okamoto, R., 2010. A comparative study of adsorption of perfluorooctane sulfonate (PFOS) onto granular activated carbon, ion-exchange polymers and non-ion-exchange polymers. Chemosphere, 80(6), pp. 647-651. DOI: 10.1016/j.chemosphere.2010.04.053 Free download from: ResearchGate
- ^ Woodard, S., Berry, J., and Newman, B., 2017. Ion exchange resin for PFAS removal and pilot test comparison to GAC. Remediation Journal, 27(3), pp. 19-27. DOI: 10.1002/rem.21515
- ^ 22.0 22.1 Guo, W., Huo, S., Feng, J., and Lu, X., 2017. Adsorption of perfluorooctane sulfonate (PFOS) on corn straw-derived biochar prepared at different pyrolytic temperatures. Journal of the Taiwan Institute of Chemical Engineers, 78, pp. 265-271. DOI: 10.1016/j.jtice.2017.06.013
- ^ 23.0 23.1 Kupryianchyk, D., Hale, S.E., Breedveld, G.D., and Cornelissen, G., 2016. Treatment of sites contaminated with perfluorinated compounds using biochar amendment. Chemosphere, 142, pp. 35-40. DOI: 10.1016/j.chemosphere.2015.04.085 Free download from: ResearchGate
- ^ 24.0 24.1 Inyang, M., and Dickenson, E.R., 2017. The use of carbon adsorbents for the removal of perfluoroalkyl acids from potable reuse systems. Chemosphere, 184, pp. 168-175. DOI: 10.1016/j.chemosphere.2017.05.161
- ^ Espana, V.A.A., Mallavarapu, M., and Naidu, R., 2015. Treatment technologies for aqueous perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA): A critical review with an emphasis on field testing. Environmental Technology and Innovation, 4, pp. 168-181. DOI: 10.1016/j.eti.2015.06.001 Free download from: ResearchGate
- ^ CETCO, 2019. FLUORO-SORB® Adsorbent (product sales brochure). Free download Fluoro-Sorb.pdf
- ^ 27.0 27.1 Zhang, Q., Deng, S., Yu, G., and Huang, J., 2011. Removal of perfluorooctane sulfonate from aqueous solution by crosslinked chitosan beads: sorption kinetics and uptake mechanism. Bioresource Technology, 102(3), pp. 2265-2271. DOI: 10.1016/j.biortech.2010.10.040
- ^ Cao, F., Wang, L., Ren, X., and Sun, H., 2016. Synthesis of a perfluorooctanoic acid molecularly imprinted polymer for the selective removal of perfluorooctanoic acid in an aqueous environment. Journal of Applied Polymer Science, 133(15). DOI: 10.1002/app.43192
- ^ 29.0 29.1 Hu, L., Li, Y., and Zhang, W., 2016. Characterization and application of surface-molecular-imprinted-polymer modified TiO2 nanotubes for removal of perfluorinated chemicals. Water Science and Technology, 74(6), pp. 1417-1425. DOI: 10.2166/wst.2016.321 Free access article.
- ^ 30.0 30.1 Zhang, C., Tang, J., Peng, C., and Jin, M., 2016. Degradation of perfluorinated compounds in wastewater treatment plant effluents by electrochemical oxidation with Nano-ZnO coated electrodes. Journal of Molecular Liquids, 221, pp. 1145-1150. DOI: 10.1016/j.molliq.2016.06.093
- ^ 31.0 31.1 Urtiaga, A., Fernández-González, C., Gómez-Lavín, S., and Ortiz, I., 2015. Kinetics of the electrochemical mineralization of perfluorooctanoic acid on ultrananocrystalline boron doped conductive diamond electrodes. Chemosphere, 129, pp. 20-26. DOI: 10.1016/j.chemosphere.2014.05.090
- ^ 32.0 32.1 Schaefer, C.E., Choyke, S., Ferguson, P.L., Andaya, C., Burant, A., Maizel, A., Strathmann, T.J. and Higgins, C.P., 2018. Electrochemical Transformations of Perfluoroalkyl Acid (PFAA) Precursors and PFAAs in Groundwater Impacted with Aqueous Film Forming Foams. Environmental Science and Technology, 52(18), pp. 10689-10697. DOI: 10.1021/acs.est.8b02726
- ^ 33.0 33.1 Park, S., Lee, L.S., Medina, V. F., Zull, A., and Waisner, S., 2016. Heat-activated persulfate oxidation of PFOA, 6: 2 fluorotelomer sulfonate, and PFOS under conditions suitable for in-situ groundwater remediation. Chemosphere, 145, pp. 376-383. DOI: 10.1016/j.chemosphere.2015.11.097
- ^ 34.0 34.1 Lin, A.Y.C., Panchangam, S.C., Chang, C.Y., Hong, P.A., and Hsueh, H.F., 2012. Removal of perfluorooctanoic acid and perfluorooctane sulfonate via ozonation under alkaline condition. Journal of Hazardous Materials, 243, pp. 272-277. DOI: 10.1016/j.jhazmat.2012.10.029
- ^ 35.0 35.1 Campbell, T., Hoffmann, M.R., 2015. Sonochemical degradation of perfluorinated surfactants: Power and multiple frequency effects. Separation and Purification Technology, 156(3), pp. 1019-1027. DOI: 10.1016/j.seppur.2015.09.053 Free download from: ResearchGate
- ^ 36.0 36.1 Cheng, J., Vecitis, C.D., Park, H., Mader, B.T., Hoffmann, M.R., 2010. Sonochemical Degradation of Perfluorooctane Sulfonate (PFOS) and Perfluorooctanoate (PFOA) in Groundwater: Kinetic Effects of Matrix Inorganics. Environmental Science and Technology, 44(1), pp. 445-450. DOI: 10.1021/es902651g
- ^ 37.0 37.1 Gole, V.L., Sierra-Alvarez, R., Peng, H., Giesy, J.P., Deymier, P., Keswani, M., 2018. Sono-chemical treatment of per- and poly-fluoroalkyl compounds in aqueous film-forming foams by use of a large-scale multi-transducer dual-frequency based acoustic reactor. Ultrasonics Sonochemistry, 45, pp. 213-222. DOI: 10.1016/j.ultsonch.2018.02.014 Open access article. Report.pdf
- ^ Gole, V.L., Fishgold, A., Sierra-Alvarez, R., Deymier, P., Keswani, M., 2018. Treatment of perfluorooctane sulfonic acid (PFOS) using a large-scale sonochemical reactor. Separation and Purification Technology, 194, pp. 104-110. DOI: 10.1016/j.seppur.2017.11.009
- ^ Cornelsen Ltd., 2015. PerfluorAd, PFC Water Treatment Solution (product sales site). Website
- ^ 40.0 40.1 Wang, Y., Lin, H., Jin, F., Niu, J., Zhao, J., Bi, Y., and Li, Y., 2016. Electrocoagulation mechanism of perfluorooctanoate (PFOA) on a zinc anode: Influence of cathodes and anions. Science of the Total Environment, 557, pp. 542-550. DOI: 10.1016/j.scitotenv.2016.03.114
- ^ 41.0 41.1 Horst, J., McDonough, J., Ross, I., Dickson, M., Miles, J., Hurst, J., and Storch, P., 2018. Water Treatment Technologies for PFAS: The Next Generation. Groundwater Monitoring and Remediation, 38(2), pp. 13-23. DOI: 10.1111/gwmr.12281
- ^ 42.0 42.1 EPC Media Group Pty Ltd., 2017. OPEC systems delivers PFAS contamination breakthrough. Waste + Water Management Australia, 44(3), 26-27. DOI: 10.3316/informit.253699294687114 ISSN: 1838-7098
- ^ 43.0 43.1 Stratton, G.R., Dai, F., Bellona, C.L., Holsen, T.M., Dickenson, E.R., and Mededovic Thagard, S., 2017. Plasma-Based Water Treatment: Efficient Transformation of Perfluoroalkyl Substances in Prepared Solutions and Contaminated Groundwater. Environmental Science and Technology, 51(3), pp. 1643-1648. DOI: 10.1021/acs.est.6b04215
- ^ 44.0 44.1 Singh, R.K., Multari, N., Nau-Hix, C., Anderson, R.H., Richardson, S.D., Holsen, T.M. and Mededovic Thagard, S., 2019. Rapid Removal of Poly- and Perfluorinated Compounds from Investigation-Derived Waste (IDW) in a Pilot-Scale Plasma Reactor. Environmental Science and Technology, 53(19), pp. 11375-11382. DOI: 10.1021/acs.est.9b02964
- ^ Xiao, F., Simcik, M.F., Gulliver, J.S., 2013. Mechanisms for removal of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) from drinking water by conventional and enhanced coagulation. Water Research, 47(1), pp. 49-56. DOI: 10.1016/j.watres.2012.09.024 Free download available from ResearchGate